Short Term Impacts of Harvesting 0perations on Soil Chemical Properties in a Mediterranean Oak Ecosystem
doi: 10.5552/crojfe.2021.1100
volume: 42, issue:
pp: 16
- Author(s):
-
- Ganatsios Harisios P.
- Tsioras Petros A.
- Papaioannou Athanasios G.
- Blinn Charles R.
- Article category:
- Original scientific paper
- Keywords:
- animal skidding, clearcutting, forest floor, thinning, organic matter, soil nutrients
Abstract
HTML
Soil physical and chemical properties can be seriously affected by forest operations. There is a knowledge gap on this topic for oak ecosystems, which can play a significant role in the context of multiple-use forestry.
The main objective of this study was to analyse forest floor and topsoil changes (0–10 cm) two years after the application of small-scale thinning (50% reduction of basal area) and clear-cut operations using mules to carry harvested material in a Northern Greece oak (Quercus frainetto Ten) ecosystem. The total amount of forest floor (O1+O2 horizons) was reduced by 37.8% in the thinned and 30.8% in the clear-cut plots compared to control plots. These large reductions are mainly due to reduction in the O2 horizon in the treated plots. Decomposition was reduced in the treated plots, possibly due to the new drier conditions. Treatments increased the soil pH but not to a significant extent. No evidence of erosion was found in the experimental plots due to the protective function of the forest floor and the use of designated mule trails. The areal extent of soil compaction was limited to only 3% of the total area mainly due to the careful planning and implementation of animal skidding. Small differences in C (%) and Ν (%) were found among control, thinned and clear-cut plots.
The limiting growth factors in Mediterranean oak ecosystems are soil depth and the seasonal change of soil moisture, especially during the summer dry period. More research on the definition of the optimum thinning degree and extraction systems in similar ecosystems will be important to satisfy the need to improve soil characteristics.
Short Term Impacts of Harvesting 0perations on Soil Chemical Properties in a Mediterranean Oak Ecosystem
Harisios P. Ganatsios, Petros A. Tsioras, Athanassios G. Papaioannou, Charles R. Blinn
Abstract
Soil physical and chemical properties can be seriously affected by forest operations. There is a knowledge gap on this topic for oak ecosystems, which can play a significant role in the context of multiple-use forestry.
The main objective of this study was to analyse forest floor and topsoil changes (0–10 cm) two years after the application of small-scale thinning (50% reduction of basal area) and clear-cut operations using mules to carry harvested material in a Northern Greece oak (Quercus frainetto Ten)ecosystem. The total amount of forest floor (O1+O2 horizons) was reduced by 37.8% in the thinned and 30.8% in the clear-cut plots compared to control plots. These large reductions are mainly due to reduction in the O2 horizon in the treated plots. Decomposition was reduced in the treated plots, possibly due to the new drier conditions. Treatments increased the soil pH but not to a significant extent. No evidence of erosion was found in the experimental plots due to the protective function of the forest floor and the use of designated mule trails. The areal extent of soil compaction was limited to only 3% of the total area mainly due to the careful planning and implementation of animal skidding. Small differences in C (%) and Ν (%) were found among control, thinned and clear-cut plots.
The limiting growth factors in Mediterranean oak ecosystems are soil depth and the seasonal change of soil moisture, especially during the summer dry period. More research on the definition of the optimum thinning degree and extraction systems in similar ecosystems will be important to satisfy the need to improve soil characteristics.
Keywords: animal skidding, clearcutting, forest floor,thinning, organic matter, soil nutrients
1. Introduction
The role of soil in forest ecosystems is of primary importance ensured by providing nutrients and water, supporting plants and their growth and regulating water yield and infiltration rates (Brady and Weil 2008). With the growing demand for wood globally (FAO 2018), there is an increased concern about the environmental impacts of forest operations on forest ecosystems and their productivity (Venanzi et al. 2016). The importance of mature forests has been gaining interest and awareness because of their unique ecosystem benefits and their loss from many landscapes (Dean and Wardell‐Johnson 2010, Gibson et al. 2011, World Resources Institute 2019). Timber harvesting can cause a loss of soil organic matter and nitrogen (Grigal 2000, Jurgensen et al. 1997), increased soil compaction and reduced porosity (Grigal 2000, Marshall 2000, Powers et al. 2005), rutting (Aust et al. 1995), reduced soil microbial activity (Hartmann et al. 2012).
There is a strong relationship between vegetative cover and soil condition. Vegetative cover exerts an important influence on soil (Grigal 2000), whereas soil condition and characteristics also determine the condition of the vegetation (Lukina et al. 2019). Soil functions that support plant growth by means of controlling plant resource availability (Schoenholtz et al. 2000) can be altered by the impacts of harvesting activities such as increased biomass removal (Slesak et al. 2017) and soil compaction (Solgi et al. 2016). As a result, understanding the impact of forest operations on soil productivity is critical. The effects of organic matter removal and soil compaction on soil properties are site-specific, which generally aligns with concepts of soil quality and its influence on vegetative growth (Slesak et al. 2017).
Soils in the Mediterranean region are particularly affected by erosion (Pereira et al. 2017) with an associated loss of topsoil. The loss of organic-rich topsoil is regarded to be the greatest degrader of soil quality and, in most cases, cannot be restored (Alewell et al. 2015). The impact of forest operations on forest soil and water continue to be an issue of concern to forest management. Research that has evaluated the impact of forest operations on forest soil and water is generally limited to mechanized operation (McFero Grace et al. 2006).
Α large number of oak ecosystems are found in Greece, varying from evergreen shrublands along the coastline to productive forests in higher elevation areas. These ecosystems are important as they constitute 44% of the total forested area of the country (Ministry of Agriculture 1992). Hungarian or Italian oak (Quercus frainetto Ten. or Quercus conferta Kit.) are the most widely distributed oak species in Greece (Konstantinidis et al. 2002) and are also native in parts of Italy, the Balkan peninsula, Hungary and Turkey (Tutin et al. 1996). The Greek Forest Service manages these oak ecosystems for both timber and non-timber ecosystem services (recreation, erosion protection, water yield production).
In Greece, the low harvest volumes per hectare and the lack of investment capital or a well-trained forest workforce currently inhibit the implementation of highly mechanized harvesting systems (Koutsianitis and Tsioras 2017). The implementation of small-scale forestry has been reported to provide both a quantitative and qualitative improvement of heavily degraded forest soils (Dafis and Kakouros 2006). This objective is imperative in Greece, given that half of the country’s total area is covered by the most important water producing areas such as forests and rangelands (Ministry of Agriculture 1992).
There is an increasing interest in the use of forested areas for improving soil characteristics such as soil carbon (Aryal et al. 2013, Alvarez and Rubio 2016), and soil nitrogen (Homyak et al. 2008). However, the literature examining the effects of different harvesting treatments in the Mediterranean region is limited (Marchi et al. 2016) and often crucial information on the harvesting systems is lacking (Cambi et al. 2015). This topic has been studied in Greece for Q. frainetto by Alifragis (1984) and Ganatsios (2004). Internationally, the impacts of thinning operations on soil have been examined much less than those of clearcutting due to many factors (e.g. more often even-aged management is used as the silvicultural method, setting of specific research goals such as examining the use of forests for flood control) (Serengil et al. 2007). Additionally, the comparability of results is difficult, if not impossible, due to the differences between the ecosystems examined and their respective attributes.
This study evaluated the effects of the use of mules to transport harvested Q. frainetto during clearcutting and thinning operations in Northern Greece on the forest floor and topsoil (0–10 cm) properties (organic C, organic N and pH) under the broader objective of improving forest soil conditions.
2. Materials and Methods
2.1 Study Area
The study was conducted in the Taxiarhis University Forest (TUF) (40°25’45.91’’ N, 23°30’18.90’’ E), managed by the Aristotle University of Thessaloniki in Greece, primarily for educational and research purposes (Fig. 1). TUF has an area of 3895 ha, is located in central Halkidiki and is dominated by hardwoods, mostly Q. frainetto. TUF is representative in terms of its topography, soil characteristics and tree species of the Mediterranean coppice stands and is also one of the most studied forests in Greece, where experiments have been carried out in many fields such as silviculture, soil science and hydrology. Its forest ecosystems are under sustainable management and support older, more mature, self-dependent ecosystems capable of producing multi-purpose ecosystem services.
The study area has a typical temperate mesothermal climate (subtype CSb), according to the Köppen classification system (Chen and Chen 2013). While moderate temperatures (average annual air temperature 11.4° C) and rainy weather prevail during the winters, summers are characterized by a short hot and dry period. Mean annual precipitation is 795 mm, which mainly falls from October to March. All climatic data are collected at the TUF weather station (860 m above sea level), located only 150 m away from the boundaries of the study area (exposure SE).
Soil parent material is vertically stratified schist (mica and talc). The soil is acidic (pH 4.5–5.2) with a subangular blocky structure. The soil belongs to Chromic Luvisols (IUSS Working Group WRB 2006) and could be described as degraded due to a history of intense erosion. More specifically, the protective vegetation cover has been removed as a result of alternating land uses in the past, including intensive wood harvesting, grazing and use of the area as agricultural land until the 1950s. After that time, the area was restored to its forest land status, and currently the soils are well-protected from erosion by vegetation and forest floor. The soil has an average depth of 45–60 cm in the upper part of the plots and is deep (>90 cm) only at the lower part of one plot. The soil is fertile but under the prevailing stand conditions, the limiting factor for forest growth is their depth and seasonal change of soil moisture. Annually, these soils are dry for more than two months during the summer period.
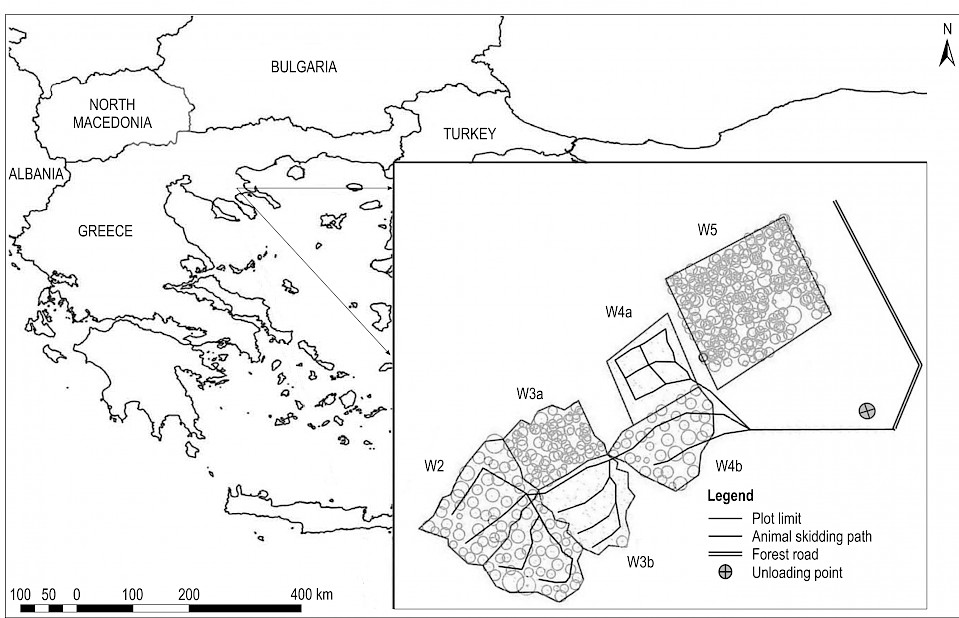
Fig. 1 Overview of study plots before and after treatment and animal trail network
The forest floor, comprised of both the O1 (litter layer composed of leaves and branches) and the O2 (decomposed organic material) horizons, has a mean depth of 4–5 cm that increases infiltration and decreases overland flow. For this reason, little soil erosion is currently evident. The H horizon (humus layer) is found below O2 and consists of well-decomposed organic material (Fisher and Binley 2000). It is thinner with a mean depth of 0.5–1 cm and, in the context of our study and for practical reasons, it has been included in O2. According to Alifragis (1984), the decomposition rate within the H horizon is high as a result of favorable temperature and moisture conditions (Alifragis 1984). Once the forest floor is removed, the topsoil characterized by a high organic matter content and a sandy clay loam texture is revealed.
2.2 Study Layout
The predominant tree species in the study plots are Q. frainetto and Fagus spp. Tree age ranges from 45 to 53 years and the forest stand is healthy with an average tree height of 16 m. Dry treetops can be witnessed in isolated instances only after dry periods. During the summer, canopy closure is 100%.
Four plots (W2 to W5), having micro-catchment characteristics (area range 1558–2000 m2) were established in the study area, based on their almost identical attributes in terms of exposure, size, shape, mix of tree species and former management actions (Fig. 1). The soil has an average depth of 45–60 cm in the upper part of the plots and is deep (>90 cm) at the lower part of only one plot. Site quality ranged between medium and good. A detailed description of the study layout is presented in Ganatsios et al. (2010).
The four plots were used for data collection for various experiments in the past. Plot W5 was previously used during a five-year study until 1984 (Alifragis 1984) and, in our case, was used as the control plot where no vegetation was removed. The upper part of W3 (W3a) served as our second control plot. A professional forester marked removal trees in the other study plots. In W2 the basal area was decreased by 50%. W3b and W4a were clearcut and in W4b, 50% of the trees were removed. Study plot characteristics are reported in Table 1.
Table 1 Study plot characteristics by treatment
|
Plot attributes |
Plots |
||||||
|
W2 |
W3a |
W3b |
W4 |
W4b |
W5 |
Total |
|
|
Treatment |
thinning |
control |
clearcut |
clearcut |
thinning |
control |
– |
|
Number of trees in plot |
241 |
107 |
124 |
85 |
85 |
365 |
1007 |
|
Tree density, trees ha-1 |
1247 |
1460 |
1503 |
1104 |
966 |
1825 |
– |
|
Basal area, m2 |
29 |
9.3 |
10 |
8 |
10 |
36 |
102.3 |
|
Standing volume, m3 ha-1 |
149 |
126.9 |
121.1 |
103.9 |
115.8 |
180 |
122.3 |
|
Number of harvested trees |
99 |
0 |
124 |
85 |
41 |
0 |
349 |
|
Harvested volume, m3 |
10.8 |
0 |
10.3 |
8 |
3.8 |
0 |
33 |
|
Area, m2 |
1946 |
733 |
825 |
770 |
864 |
2000 |
7138 |
|
Slope range, % |
10–30 |
5–20 |
5–20 |
0–10 |
0–10 |
0–5 |
– |
Harvesting operations in TUF are practiced motor-manually by a forest workers’ cooperative and are closely supervised by the Greek Forest Service. As described in the management plan, all thinning operations aim to convert the existing coppice into a high quality forest (Taxiarhis University Forest 1991).
All harvesting operations were conducted by a team of three forest workers who used chainsaws for tree felling, delimbing and bucking in October 2001. The forest workers had an average of 12 years of experience felling, delimbing and bucking in thinnings and clearcut areas. Once severed from the tree, processed firewood and thicker branches were bunched together and loaded on two mules, which were also used for log extraction (Fig. 2). The mules always stayed on trails where they were loaded to carry around 200 kg prior to transporting their load to a centralized location. The forest workers were instructed to minimize the number of skidding cycles (each cycle consisted of empty travel, loading, loaded travel and unloading) and to use pre-existing animal skidding trails to minimize the area affected by soil compaction. Fine woody debris, in the form of branches with a maximum diameter of 10 cm (Nordén et al. 2004) were removed from the study plots and manually dispersed to adjacent forested areas outside of the study plots. Only the stump and the root system of the harvested trees remained in the forest in order to facilitate the comparison between treatments. The in-woods harvest process was closely monitored by the study team.

Fig. 2 Manual felling and processing with chainsaw (a), wood extraction by mules (b), loaded travel (c) and unloading point (d)
2.3 Sampling and Data Collection
Prior to implementing the harvest treatments, baseline data was collected. These data included an inventory of all the trees in the four plots with their exact position recorded using a GPS, their DBH and total tree height. Our study focused on changes at the soil depth of 0–10 cm, because the upper portion of the topsoil is more susceptible to observed changes after harvesting treatments in Mediterranean ecosystems (Gakis et al. 2003). Furthermore, soil organic C and N concentrations were measured as they are important regulating factors of microbial activity and soil fertility (Salmon 2018).
Systematic sampling was carried out with the use of a sampling grid (Köhl and Magnussen 2016). Thirty soil sampling points were designated in each of the plots W2–W5, or 120 in total. A square frame (25×25 cm) was used to delimit the sampling points where:
Þ all the forest floor was collected
Þ soil samples from depths of 0–2, 2.1–5 and 5.1–10 cm were put in separate plastic bags, labeledand transported to the laboratory for analysis. The sampling process was repeated two years after the harvesting treatments.
The network of animal skidding trails was mapped with a measuring tape and GPS coordinates were taken with a Garmin eTrex® handheld device after the wood extraction was completed. Furthermore, the width of the trails was measured every 5 m in order to determine their areal extent prior to and after harvest.
2.4 Laboratory Measurements
Forest floor samples were dried at 84°C until a constant weight was achieved and weighed in the laboratory. All mineral soil samples were air dried and sieved through 2 mm mesh screens and stored in numbered boxes.
Sample pH was determined using a glass electrode on a soil/water suspension (1:1, by weight) (Miller and Kissel 2010, Kavvadias et al. 2001). Soil organic carbon was determined by means of wet oxidation (Papaioannou 2013). Finally, organic N was determined using the Kjeldahl method (Stevenson 1982).
2.5 Soil Erosion
Soil erosion was measured by means of graduated metal pins (Myers et al. 2019). Ten erosion pins were randomly inserted in each plot before the harvesting operations and were examined two years after.
2.6 Statistical Analysis
One-way ANOVA was used to assess the significance of observed differences in means. Tukey’s HSD post-hoc test was used to identify homogeneous groups of means among different treatments and soil depths (Zar 1999). All statistical analyses were conducted with the SPSS software version 23 and R version 3.5.1 (R Core Team 2018). Significance level was set at 0.05.
3. Results
A total of 349 trees were harvested during the study resulting in 33 m3 of firewood produced (Table 1). A thinning rate of 37% of the standing volume was applied to thinned plots. The number of harvested trees per plot ranged from 41 to 124, depending on the basal area and the treatment type. No deviations from the guidelines given to the forest workers were observed by the study team.
3.1 Forest Floor Changes
The results of the forest floor change two years after treatment application are presented in Table 2. Data analysis revealed significant differences (F=27.759, p<0.0001) among the treatments in the O2 horizon, where the control plots (15,316 kg ha-1) were characterized by 45.7% more forest floor than the thinned (8206 kg ha-1) and 56.6% more than the clearcut (6558 kg ha-1) plots. On the contrary, no significant differences (F=1.533, p=0.219) were identified among the treatments in the O1 horizon, where the mean values ranged from 4749 to 5372 kg ha-1. As in O2, the amount of O1 in the forest floor within the thinning treatment was found to be higher compared to the clearcut plots.
Table 2 Forest floor weight (kg ha-1) and O2/O1 ratio in study treatments two years after harvest operations
|
Variable |
Control |
Thinning |
Clearcut |
|||
|
Before treatment |
2 years after treatment |
Before treatment |
2 years after treatment |
Before treatment |
2 years after treatment |
|
|
O1 |
5238 a |
5372 a |
5846 a |
4815 a |
5151 a |
4749 a |
|
O2 |
15,118 a |
15,316 a |
15,097 a |
8206 b |
11,177 b |
6558 b |
|
O1+O2 |
20,356 a |
20,688 a |
20,942 a |
13,021 b |
16,328 b |
11,307 b |
|
O2 / O1 |
2.88 a |
2.85 a |
2.58 a |
1.70 b |
2.17 b |
1.38 b |
Similarly, the total amount of forest floor (O1+O2) was found to be significantly different among the three treatments two years after the harvest operations (F=24.743, p<0.0001) following the considerable changes in O2. More specifically, O1+O2 was found to have, in descending order, its highest value in the control plots (20,688 kg ha-1), the thinned plots (13,021 kg ha-1) and finally in the clearcut plots (11,307 kg ha-1) (Table 2).
In our study, the O2/O1 ratio was 2.85 in the control plots and decreased to 1.70 and 1.38 in the thinned and clearcut plots, respectively (Table 2). These differences were significant (F=10.546, p<0.0001).
3.2 Surface Soil Changes
The results of the soil sampling analysis for pH and organic C (%) and organic N (%) concentrations are included in Table 3. We found that pH decreased with increasing soil depth. Soil pH ranged from 5.019 to 5.119 and from 4.830 to 4.988 at soil depths of 0–2 cm and 5.1–10 cm, respectively. At all depths (0–2, 2.1–5 and 5.1–10 cm), the pH values of clearcut plots were significantly higher than those of the other two treatments with the exception of the thinning treatment at the depth of 5.1–10 cm.
Table 3 Mean values of pH, organic C (%), organic N (%), and C/N ratio per soil depth and treatment two years after the harvest operations
|
Variable |
pH |
C, % |
||||
|
0–2 |
2.1–5 |
5.1–10 |
0–2 |
2.1–5 |
5.1–10 |
|
|
cm |
||||||
|
Control |
5.019 a |
4.997 a |
4.830 a |
4.944 a |
3.977 a |
3.747 a |
|
Thinning |
5.078 a |
5.020 a |
4.940 ab |
5.104 a |
4.427 b |
3.924 ab |
|
Clearcut |
5.119 b |
5.089 b |
4.988 b |
5.177 a |
4.870 b |
4.160 b |
|
Variable |
N, % |
C/N |
||||
|
0–2 |
2.1–5 |
5.1–10 |
0–2 |
2.1–5 |
5.1–10 |
|
|
cm |
||||||
|
Control |
0.451 a |
0.416 a |
0.302 a |
10.96 a |
9.56 a |
12.40 a |
|
Thinning |
0.314 b |
0.263 b |
0,187 b |
16.25 b |
16.83 b |
20.98 b |
|
Clearcut |
0.257 c |
0.221 c |
0.168 b |
20.14 c |
22.03 c |
24.76 b |
The concentration of organic C decreased with increasing soil depth. In all treatments and starting from the 0–2 cm depth, C values ranged from 4.944 to 5.177%, from 3.977 to 4.870% for the 2.1–5 cm depth, and from 3.747 to 4.160% at the depth of 5.1–10 cm (Table 3). The decrease of organic C was significantly different only at the depths of 2.1–5 cm (F=44.382, p<0.0001) and 5.1–10 cm (F=6.384, p<0.0001) among the three treatments. At all soil depths, the largest concentration of organic C was found in the clearcut treatment followed by the thinning and control treatments.
Similar trends were noted for organic N, whose concentration decreased with increasing soil depth. Organic N values ranged from 0.257 to 0.451% within the 0–2 cm depth, 0.416 to 0.221% for the 2.1–5 cm depth, and 0.168 to 0.302% at the depth of 5.1–10 cm (Table 3). The concentration of organic N was significantly different among all treatments with the exception of the 5.1–10 cm. At all soil depths, the largest concentration of organic N was measured in the control treatment followed by the thinning and clearcut treatments.
At the 0–2 cm soil depth, the C/N ratio differed among the treatments ranging from 10.96 to 20.14 (F=1133.907, p<0.0001) (Table 3). The lowest ratio (9.56) was recorded in the control plot within the 2.1–5 cm depth (F=1341.897, p<0.0001). The thinning and clearcut treatments had C/N ratios that were 64.39% and 104.47.1% higher, respectively, than the control treatment. Significant differences between thinning and clearcut C/N treatment means were not found at the 5.1–10 cm soil depth (F=1.497, p=0.249).
3.3 Areal Extent of Soil Compaction and Surface Erosion
The harvesting system used in the study imposed very limited influence on soil compaction. Animal skidding was limited to the existing trails that covered less than 3% of the study area. The majority of the trails were used for only two skidding cycles during the wood extraction phase of the operation, with the exception of the central trail that led to the drop-off point, close to the forest road. Furthermore, the trail width remained unchanged apart from a very limited number of cases. However, even in these cases the increase was minimal and hard to identify.
The most intense rain incident during the study period was recorded one month before the treatments were applied (49 mm in 3 h). No erosion was observed post-harvest because the soil was dry and ground vegetation was retained intact during the harvesting operation. No displacement of the forest floor on the surface soil was observed either at the sampling spots or on the animal trails. As expected, the forest floor acted as a protective shield.
4. Discussion
4.1 Change in Organic Matter
Skidding operations may lead to considerable immediate reductions of organic matter, depending on the slope, traffic intensity and equipment used. Such reductions may be up to 64% (Naghdi et al. 2016b) and 93% (Naghdi et al. 2016a) of the organic matter on the skid trails, as reported by two studies in hardwoods in Northern Iran. The forest floor dry weight (O1+O2) was 36% lower in the thinned plots and 44.5% lower in the clearcut plots as compared to the control plots. Marchi et al. (2016) reported similar results with the amount of organic matter being higher in the control area than in other treatments after examining the impacts of logging operations in Mediterranean Quercus cerris coppice stands with applicable standards. In North America, Hix and Barnes (1984) reported a 35% reduction of organic matter weight in mixed stands 46 years after applying the treatments, while Briggs et al. (2000) reported a 47% reduction in conifer stands four years after treatment application. In other studies, the amount of organic matter increased after applying logging treatments in hardwoods in North America (Johnson et al. 1991, Yanai et al. 2000). However, the results are not directly comparable due to the different tree species, site and stand conditions and the time span between the harvesting treatment and data collection.
For the control plot W5, Alifragis (1984) reported a forest floor dry weight (O1+O2) of 13,368 kg ha-1 as well as a net input of organic matter to the forest floor of 917 kg ha-1 for a period of two years. At that time, trees were 34 years old. Our reported mean value for the control plots was 20,688 kg ha-1. During the harvesting operations in this study, all branches were removed from the plots, and the biomass was equivalent to 5732 kg ha-1, which is comparable to the initial amount of O1 horizon (5372 kg ha-1) in the control plots. The reduction of organic material can be attributed to the lower input of foliar litter on the ground in the two years after the harvest occurred (Alifragis 1992, Brix 1981, Klemmedson 1987) as well as the increased decomposition rate of the forest floor (Alifragis 1992). In the O1 horizon of the thinned and clearcut plots, the reduction was limited (8.1% and 9.3%, respectively). According to Wallace and Freedman (1986), the input changes of organic material on the forest floor after harvesting hardwoods in Canada are more important than the changes in decomposition rates.
The control plot was characterized by 45.7% more O2 horizon than the thinned and 56.6% more than the clearcut plots. These considerable differences suggest the large impact of harvesting treatments on the removal of O2. Reports of forest floor reduction after hardwood logging in North America are very common, especially when mechanized skidding equipment is used, due to the mixing of the O2 horizon with inorganic soil (Huntington and Ryan 1990, Mroz et al. 1985, Ryan et al. 1992, Yanai 1998). The use of machine skidding equipment was forbidden in TUF in order to minimize adverse impacts of harvesting treatments to the residual stands. The overall level of harvest mechanization in Greece is still relatively low (Tsioras 2012) but is increasing due to the declining number of forest workers. Thus, the reduction in forest floor in Greece due to the use of skidding machinery should be addressed in future studies.
Harvesting leads to a significant reduction of soil organic matter (Jurgensen et al. 1997). The reduction of organic matter found in the current study suggests higher decomposition rates in thinned plots as compared to clearcut plots. Thinning, in general, is considered to be a tool for improving decomposition, humification and mineralization in forest soils (Grigal 2000, Swank and Webster 2014, Yanai et al. 2000). However, intense canopy reduction (40% of basal area removed) reduced decomposition rates of oak foliar litter under Mediterranean conditions (Bravo-Oviedo et al. 2017). Such dissimilarities to decomposition rates may be attributed to different altitude and climate. Furthermore, the thickness and the quantity of forest floor varies according to the season (Paudel et al. 2015).
As the amount of forest floor is higher in mature forests than in young or middle-aged forests (Yanai et al. 2000), the harvesting system applied will have differential impacts on the forest floor and soil disturbance. Motor manual harvesting operations have been reported to cause less soil disturbance as compared to the use of heavy machinery (Naghdi et al. 2015, Wang 1997, Spinelli et al. 2014). Wood extraction methods can be ranked in order of decreasing impact to soil: whole tree skidding, tree length skidding/shortwood forwarding, tree length cable crane, and shortwood cable crane (Worrell and Hampson 1997). Another important, but not frequently discussed, issue is the impact of the human factor on the quality of harvesting operations and thus the site. The magnitude of environmental impacts is to a large extent influenced by the responsible workforce (Tsioras and Liamas 2015), whose limited skills may pose an additional threat to soil and residual stand.
4.2 O2/O1 Ratio
Analysis of the O2/O1 ratio can provide useful information on the decomposition rate of the organic material. More specifically, lower O2/O1 ratios suggest lower decomposition rates. The highest, almost similar, O2/O1 ratios were observed in the control plots before (2.88) and two years after harvesting (2.85), respectively. This small reduction could be attributed to the proximity of one of the two control plots (W3a) to W3b (clearcut plot) and the improved decomposition conditions due to incoming solar radiation on the soil surface after the clearcut treatment (Coulombe et al. 2017, Papaioannou 2015). On the contrary, the lowest O2/O1 ratios were observed two years after treatment in the thinned (1.70) and clearcut plots (1.38), suggesting the importance of the harvesting intensity on the decomposition rate. Generally, the wide range of the O2/O1 ratio may be attributed to site-specific factors, such as the forest soil type, decomposition conditions of organic material and forest stand conditions (Kavvadias et al. 2001). Similar results of increased decomposition rates have been reported during the management of chestnut forests at Mountain Athos in Greece (Papaioannou 2013).
Based on the previous study in the area by Alifragis (1984), 75% of O1 should have been completely decayed within 24 months after logging. Nevertheless, that result was not validated by our findings as the differences regarding the amount of O1 among control, thinned and clearcut treatments were limited.
In the control plots, the weight of the O2 horizon was approximately three times larger than that of the O1 horizon (Table 2), whereas in the thinning and clearcut treatments the weight of the O2 horizon was almost twice as much as in O1, where there was limited reduction in the O1 horizon. The reduction of O2 within the thinning and clearcut treatments may result from a deterioration of decay conditions created by the removal of canopy.
Woody debris preserves the forest floor which regulates soil temperature and moisture conditions (Bonanomi et al. 2019). Additionally, detrital thickness is responsible for creating a more balanced microclimate for soil organisms (Fekete et al. 2016). The treatments applied in this study removed all harvested coarse wood. After intense logging, as in our case with the thinning and clearcut treatments, reduction of decomposition rates for broadleaved ecosystems within the temperate zone has been reported (Remezov and Pogrebnyak 1969). Temperature is considered a prime factor in determining the rates of litter decomposition (Krishna and Mohan 2017). Drought, especially in the early stages of decomposition, can limit microbial activity; later on, litter has an increased ability to retain water (De Santo et al. 1993). Similar or lower decomposition rates in clearcut areas have also been observed and attributed to the negative effects of temperature extremes and drier surface conditions on microbial activity (Prescott 1997, Palviainen et al. 2004).
4.3 pH, C, N and C/N Changes in the Topsoil
In our study, harvesting increased pH up to a 10 cm depth. pH was found highest in clearcut plots (pH=5.04), followed by thinned plots (pH=4.99) and, finally, by the control plots (pH=4.92). This might be correlated to the increased precipitation measured in the area during the study period (Ganatsios et al. 2010). Soil pH is affected by many factors. Canopy exerts an impact by changing acid conditions of throughfall water (Kelly and Strickland 1986) as well as tree trunks during stemflow (Crozier and Boerner 1986). In cold and wet climates, acid production resulting from decomposition of organic residues and humification can reduce pΗ (Pritchett 1979). Nonacid cations are depleted from the soil (or exchangeable complex) during decomposition as they are replaced by acid cations (Alifragis 1984).
Marchi et al. (2016) reported that soil pH was not influenced by silvicultural treatments or harvesting in a Q. cerris coppice ecosystem in Italy. On the contrary, canopy presence in P. maritima plots in Greece reduced soil pH from 5.17 to 5, while thinning increased it from 5 to 5.12 (Papamichos and Alifragis 1990).
According to the study results (Table 3), the differences in pH, C (%) and Ν (%) were small across the treatments. Kurth et al. (2014) reported that stem-only harvesting effects on soil C and N concentrations are site specific and more apparent in the forest floor than the mineral soil in northern U.S. poplar stands. Forest harvesting, on average, has little or no effect on soil C and N (Johnson and Curtis 2001). Nevertheless, in our study, thinning and clearcut treatments temporarily increased C and N content. The highest value across all studied soil depths was observed in clearcut plots, which serves as an indication of relatively good humification conditions. Mineralization of organic C and nitrification are indicators of forest ecosystem health and evolution (Morris and Boerner 1998). Mechanized skidding has been reported to cause unfavorable changes in soil pH and nutrient status, jeopardizing the sustainability of forest ecosystems (Naghdi et al. 2016b).
The C/N ratio is recognized as a good indication of the decomposition rate of organic matter and available N (Ross et al. 2011, Yamakura and Sahunalu 1990). Examination of the topsoil C/N ratio changes shows rising values with increasing soil depth in the case of thinning and clearcutting. The only exception (reduction) was found in the soil depth of 2.1–5 cm in the control plot. This is an unexpected result, as the C/N ratio usually decreases with depth (Dietzel et al. 2017, Marty et al. 2017). The C/N ratio was reported to change with the treatment type, becoming lower as decomposition proceeded and nitrification increased in pine stands of southern U.S. (Vitousek and Matson 1985). Initially, thinning improves the C/N ratio, the related decomposition conditions and, therefore, the mobilization of nutrients (Vitousek and Matson 1985, Alifragis 1992). Schmitz et al. (1998) reported the same conclusion after clearcutting operations.
4.4 Surface Erosion and Compaction
No signs of soil erosion were found during the study period, possibly as a result of the combination of the use of animals for carrying out harvested material and the ability of the soil to minimize stormflow events. With regard to soil compaction, the choice of mules and use of pre-established trails minimized the affected area to only 3% of the total area, very close to the finding of 3.4±0.9% by Marchi et al. (2016), 4.3% by Jourgholami and Majnounian (2013) and 5% by (Wang 1999).
The use of mechanized equipment, especially the tree-length and full-tree systems where material is dragged along the ground, often results in a larger area of the harvest site impacted, more soil surface exposure and more compaction as compared to animal skidding. The areal extent of soil disturbance during mechanized operations can be as high as 40% (Nikooy et al. 2020, Tavankar et al. 2017, Zenner et al. 2007). The severity of soil disturbance during animal skidding has been reported to be lower as compared to using mechanized harvesting equipment (Shrestha et al. 2008). Naghdi et al. (2015) reported that, while the use of rubber-tired skidders and a crawler skidder caused apparent compaction in mature hardwood forests in Iran, mule hauling did not cause that disturbance. Wang (1997) reported that animal skidding caused significantly less soil disturbance and damage to residual stands and seedlings as compared to heavy machine skidding in selective cutting and thinning operations. Wang (1997) also suggested that animal skidding is the preferred alternative among all skidding alternatives in the moderate and steep terrain conditions of the Heilongjiang Forest Region in China.
While animal harvesting has been reported to cause less soil disturbance than mechanized equipment, it is more labor intensive. Logging is physically demanding and can be dangerous with workers spending time outdoors, often in isolated areas (Bureau of Labor Statistics 2020). Analogous harsh working conditions have been associated with high prevalence of musculoskeletal disorder symptoms (Dimou et al. 2020). As Europe’s workforce continues to age (International Labour Organization 2018), many members of Generation Z want to stay connected with others through social media, working for companies which closely align with their own values (Blinn 2019). Logging businesses that have difficulty in recruiting and retaining quality labor may become more mechanized to maintain their level of production (Tsioras 2010). In this context, the combination of teleoperation with winch-assist technology (Cavalli and Amishev 2019) could provide a viable solution for Greek forestry which is characterized by steep terrain.
Another possible reason for the limited extent of soil compaction is the use of pre-planned animal trails, as was done in the hardwood forests of Iran (Jamshidi et al. 2008). This point underlines the importance of planning the layout of in-woods trails before the harvest, communicating that plan to forest workers, and having a well-qualified forest workforce who understand the plan and its importance in reducing the impacts of harvesting operations on the site and residual stand (Nikooy et al. 2010). In addition, supervision of the ongoing harvest operation could help ensure the implementation of the trail design.
5. Conclusions
This study focuses on the use of small-scale forest operations within the widely distributed but not extensively examined Mediterranean oak ecosystems. Specifically, it provides insight into the effects of different intensity harvesting operations with mules on topsoil properties in an oak ecosystem in Greece. Topsoil was affected in various ways, starting with a significant reduction of organic residues on the forest floor (O1 and O2 horizons). In all treatments, the volume of the O2 horizon was higher compared to the O1 horizon, which is an indication of relatively good decomposition conditions of organic matter. Furthermore, decomposition in the thinned plots was much higher in comparison to the clearcut plots mainly due to the new warmer and drier conditions of the forest floor in the clearcut plots. Also, the rate of organic matter accumulation and the amount and weight of forest floor in the control plots was higher compared to the other treatments.
Soil pH increased with increasing thinning intensity. Organic C and N decreased with increasing soil depth in all treatments. The differences in pH, C (%) and Ν (%) among the control, thinned and clearcut plots were very limited.
The limiting factor for forest growth in these soils is the soil depth and water availability during summer drought. Maintaining the forest floor is essential in regulating temperature and moisture conditions and enriching the soil with nutrients from leaves and woody debris. Sustainable forest management can benefit Mediterranean ecosystems to a considerable degree. Similarly, increasing the soil depth by a few cm might be crucial for forest growth or even survival during dry seasons.
Increasing the awareness of and knowledge about the interconnections between sustainably managed ecosystems and timber harvesting operations is important. The objectives of increasing wood and water yield production in the long term can be achieved with low intensity thinning treatments that will also improve soil properties of similar Mediterranean ecosystems. The careful implementation of wood extraction with animals was responsible for the limited impacts on the forest floor and soil in the study plots compared to the use of heavier equipment. However, the option of using animals for wood extraction may not be available in the coming years due to the declining size of the forestry workforce. In this context, additional research is necessary, especially with regard to the intensity and the long-term effects of thinning treatments and the development and testing of low-cost and low environmental impact forest machinery.
Acknowledgements
The authors wish to thank Prof. D. Alifragis for the data he provided from his previous research and his support and guidance during the study.
6. References
Alewell, C., Egli, M., Meusburger, K., 2015: An Attempt to Estimate Tolerable Soil Erosion Rates by Matching Soil Formation with Denudation in Alpine Grasslands. J Soils Sediments 15(6): 1383–1399. http://doi.org/10.1007/s11368-014-0920-6
Alifragis, D., 1984: Nutrient Dynamics and Organic Matter Production in an Oak Ecosystem. PhD Thesis. Aristotle University of Thessaloniki, Thessaloniki, 115 p.
Alifragis, D., 1992: Effects Thinning and Fertilization of Forest Litter of Pinus Maritima. Scientific Annals of the School of Forestry and Natural Environment LE/2(23): 721–743.
Alvarez, S., Rubio, A., 2016: Wood Use and Forest Management for Carbon Sequestration in Community Forestry in Sierra Juárez, Mexico. Small-scale Forestry 15(3): 357–374. http://doi.org/10.1007/s11842-016-9325-2
Aryal, S., Bhattarai, D.R., Devkota, R.P., 2013: Comparison of Carbon Stocks between Mixed and Pine-Dominated Forest Stands within the Gwalinidaha Community Forest in Lalitpur District, Nepal. Small-scale Forestry 12(4): 659–666. http://doi.org/10.1007/s11842-013-9236-4
Aust, W.M., Tippett, M.D., Burger, J.A., McKee, W.H., 1995: Compaction and Rutting During Harvesting Affect Better Drained Soils More Than Poorly Drained Soils on Wet Pine Flats. South J Appl For 19(2): 72–77. http://doi.org/10.1093/sjaf/19.2.72
Blinn, C.B., 2019: Assessing Concerns of Logging Business Owners in the Lake States Region. Forest Resoures Association. Technical Release 19-R-13, 5 p.
Bonanomi, G., De Filippis, F., Cesarano, G., La Storia, A., Zotti, M., Mazzoleni, S., Incerti, G., 2019: Linking Bacterial and Eukaryotic Microbiota to Litter Chemistry: Combining Next Generation Sequencing with 13c Cpmas Nmr Spectroscopy. Soil Biology and Biochemistry 129: 110–121. http://doi.org/10.1016/j.soilbio.2018.11.013
Brady, N.C., Weil, R.R., 2008: The Nature and Properties of Soil. 14 ed.; Prentice Hall: Upper Saddle, NJ, ISBN 013227938X, 980 p.
Bravo-Oviedo, A., Ruiz-Peinado, R., Onrubia, R., del Río, M., 2017: Thinning Alters the Early-Decomposition Rate and Nutrient Immobilization-Release Pattern of Foliar Litter in Mediterranean Oak-Pine Mixed Stands. For Ecol Manage 391: 309–320. http://doi.org/10.1016/j.foreco.2017.02.032
Brix, H., 1981: Effects of Thinning and Nitrogen Fertilization on Branch and Foliage Production in Douglas-Fir. Can J For Res 11(3): 502–511. http://doi.org/10.1139/x81-069
Bureau of Labor Statistics. Available online: https://www.bls.gov/ooh/farming-fishing-and-forestry/logging-workers.htm
Cambi, M., Certini, G., Neri, F., Marchi, E., 2015: The Impact of Heavy Traffic on Forest Soils: A Review. For Ecol Manage 338: 124–138. http://doi.org/10.1016/j.foreco.2014.11.022
Cavalli, R., Amishev, D., 2019: Steep Terrain Forest Operations – Challenges, Technology Development, Current Implementation, and Future Opportunities. International Journal of Forest Engineering 30(3): 175–181. http://doi.org/10.1080/14942119.2019.1603030
Chen, D., Chen, H.W., 2013: Using the Köppen Classification to Quantify Climate Variation and Change: An Example for 1901–2010. Environmental Development 6: 69–79. http://doi.org/10.1016/j.envdev.2013.03.007
Coulombe, D., Sirois, L., Paré, D., 2017: Effect of Harvest Gap Formation and Thinning on Soil Nitrogen Cycling at the Boreal – Temperate Interface. Can J For Res 47(3): 308–318. http://doi.org/10.1139/cjfr-2016-0301
Crozier, C.R., Boerner, R.E.J., 1986: Stemflow Induced Soil Nutrient Heterogeneity in a Mixed Mesophytic Forest. Bartonia (52): 1–8.
Dafis, S., Kakouros, P., 2006: Guidelines for the Rehabilitation of Degraded Oak Forests. Greek Biotope/Wetland Centre: Thermi, Greece, 41 p. http://doi.org/10.13140/2.1.2062.1448
De Santo, A.V., Berg, B., Rutigliano, F.A., Alfani, A., Floretto, A., 1993: Factors Regulating Early-Stage Decomposition of Needle Litters in Five Different Coniferous Forests. Soil Biology and Biochemistry 25(10): 1423–1433. http://doi.org/10.1016/0038-0717(93)90057-I
Dean, C., Wardell‐Johnson, G., 2010: Old‐Growth Forests, Carbon and Climate Change: Functions and Management for Tall Open‐Forests in Two Hotspots of Temperate Australia. Plant Biosystems – An International Journal Dealing with all Aspects of Plant Biology 144(1): 180–193. http://doi.org/10.1080/11263500903560751
Dietzel, R., Liebman, M., Archontoulis, S., 2017: A Deeper Look at the Relationship between Root Carbon pools and the Vertical Distribution of thesoil carbon pool. Soil 3(3): 139–152. http://doi.org/10.5194/soil-3-139-2017
Dimou, V., Malesios, C., Pispa, S., 2020: Monitoring Self-Reported Musculoskeletal Symptoms in Forestry Operations. International Journal of Forest Engineering 31(2): 106–113. http://doi.org/10.1080/14942119.2020.1745530
FAO. 2018: The State of the World’s Forests 2018 – Forest Pathways to Sustainable Development. Rome, Italy, 118 p.
Fekete, I., Varga, C., Biró, B., Tóth, J.A., Várbíró, G., Lajtha, K., Szabó, G., Kotroczó, Z., 2016: The Effects of Litter Production and Litter Depth on Soil Microclimate in a Central European Deciduous Forest. Plant Soil 398(1): 291–300. http://doi.org/10.1007/s11104-015-2664-5
Fisher, R.F., Binley, D., 2000: Ecology and Management of Forest Soils. 3rd; John Wiley & Sons, Inc.: New York, 489 p.
Gakis, S., Papaioannou, A., Tantos, V., Kostakis, S., 2004: Organic matter and nutrients accumulation on the surface soil of Abies borisii-regis Mattf. and Pinus nigra Arn. stands. In Proceedings of an international scientific conference marking 75 years of the Forest Research Institute of the Bulgarian Academy of Sciences, Forest Research Institute. Sofia, Bulgaria, 1–5 October 2003. Vol. 2: 295–299.
Ganatsios, H.P., 2004: Interactions between Harvesting Systems and Forest Ecosystems. PhD Thesis. Aristotle University of Thessaloniki, Thessaloniki, 147 p.
Ganatsios, H.P., Tsioras, P.A., Pavlidis, T., 2010: Water Yield Changes as a Result of Silvicultural Treatments in an Oak Ecosystem. For Ecol Manage 260(8): 1367–1374. http://doi.org/10.1016/j.foreco.2010.07.033
Gibson, L., Lee, T.M., Koh, L.P., Brook, B.W., Gardner, T.A., Barlow, J., Peres, C.A., Bradshaw, C.J.A., Laurance, W.F., Lovejoy, T.E., Sodhi, N.S., 2011: Primary Forests Are Irreplaceable for Sustaining Tropical Biodiversity. Nature 478(7369): 378–381. http://doi.org/10.1038/nature10425
Grigal, D.F., 2000: Effects of Extensive Forest Management on Soil Productivity. For Ecol Manage 138(1–3): 167–185. http://doi.org/10.1016/s0378-1127(00)00395-9
Hartmann, M., Howes, C.G., VanInsberghe, D., Yu, H., Bachar, D., Christen, R., Henrik Nilsson, R., Hallam, S.J., Mohn, W.W., 2012: Significant and Persistent Impact of Timber Harvesting on Soil Microbial Communities in Northern Coniferous Forests. The ISME journal 6(12): 2199–2218. http://doi.org/10.1038/ismej.2012.84
Hix, D.M., Barnes, B.V., 1984: Effects of Clear-Cutting on the Vegetation and Soil of an Eastern Hemlock Dominated Ecosystem, Western Upper Michigan. Can J For Res 14(6): 914–923. http://doi.org/10.1139/x84-163
Homyak, P.M., Yanai, R.D., Burns, D.A., Briggs, R.D., Germain, R.H., 2008: Nitrogen Immobilization by Wood-Chip Application: Protecting Water Quality in a Northern Hardwood Forest. For Ecol Manage 255(7): 2589–2601. http://doi.org/10.1016/j.foreco.2008.01.018
Huntington, T.G., Ryan, D.F., 1990: Whole-Tree-Harvesting Effects on Soil Nitrogen and Carbon. For Ecol Manage 31(4): 193–204. http://doi.org/10.1016/0378-1127(90)90067-L
International Labour Organization. Available online: https://www.ilo.org/brussels/information-resources/news/WCMS_645926/lang--en/index.htm
IUSS Working Group WRB 2006: World Reference Base for Soil Resources 2006. FAO: Rome, Italy. http://www.fao.org/fileadmin/templates/nr/images/resources/pdf_documents/wrb2007_red.pdf 17 p.
Jamshidi, R., Jaeger, D., Raafatnia, N., Tabari, M., 2008: Influence of Two Ground-Based Skidding Systems on Soil Compaction under Different Slope and Gradient Conditions. International Journal of Forest Engineering 19(1): 9–16. http://doi.org/10.1080/14942119.2008.10702554
Johnson, C.E., Johnson, A.H., Huntington, T.G., Siccama, T.G., 1991: Whole-Tree Clear-Cutting Effects on Soil Horizons and Organic-Matter Pools. Soil Sci Soc Am J 55(2): 497–502. http://doi.org/10.2136/sssaj1991.03615995005500020034x
Johnson, D.W., Curtis, P.S., 2001: Effects of Forest Management on Soil C and N Storage: Meta Analysis. For Ecol Manage 140(2): 227–238. http://doi.org/10.1016/S0378-1127(00)00282-6
Jourgholami, M., Majnounian, B., 2013: Traditional Mule Logging Method in Hyrcanian Forest: A Study of the Impact on Forest Stand and Soil. Journal of Forestry Research 24(4): 755–758. https://doi.org/10.1007/s11676-013-0370-9
Jurgensen, M.F., Harvey, A.E., Graham, R.T., Page-Dumroese, D.S., Tonn, J.R., Larsen, M.J., Jain, T.B., 1997: Impacts of Timber Harvesting on Soil Organic Matter, Nitrogen, Productivity, and Health of Inland Northwest Forests. Forest Sci 43(2): 234–251. http://doi.org/10.1093/forestscience/43.2.234
Kavvadias, V.A., Alifragis, D., Tsiontsis, A., Brofas, G., Stamatelos, G., 2001: Litterfall, Litter Accumulation and Litter Decomposition Rates in Four Forest Ecosystems in Northern Greece. For Ecol Manage 144(1): 113–127. http://doi.org/10.1016/S0378-1127(00)00365-0
Kelly, J.M., Strickland, R.C., 1986: Throughfall and Plant Nutrient Concentration Response to Simulated Acid Rain Treatment. Water, Air, and Soil Pollution 29(3): 219–231. http://doi.org/10.1007/BF00158755
Klemmedson, J.O., 1987: Influence of Oak in Pine Forests of Central Arizona on Selected Nutrients of Forest Floor and Soil. Soil Sci Soc Am J 51(6): 1623–1628. http://doi.org/10.2136/sssaj1987.03615995005100060039x
Köhl, M., Magnussen, S., 2016: Sampling in Forest Inventories. In: Tropical Forestry Handbook; Pancel, L., Köhl, M. Eds; Springer Berlin Heidelberg: Berlin, Heidelberg, 777–837 p.
Konstantinidis, P., Chatziphilippidis, G., Tsiourlis, G., Tsiontsis, A., 2002: Taxonomy and Ecology of Plant Communities of Quercus frainetto Ten. (Q. conferta Kit.) Forests in Greece. Isr J Plant Sci 50(2): 145–154. http://doi.org/10.1560/45PR-X4MC-CLYG-CGVJ
Koutsianitis, D., Tsioras, P.A., 2017: Time Consumption and Production Costs of Two Small-Scale Wood Harvesting Systems in Northern Greece. Small-scale Forestry 16(1): 19–35. http://doi.org/10.1007/s11842-016-9340-3
Krishna, M.P., Mohan, M., 2017: Litter Decomposition in Forest Ecosystems: A Review. Energy, Ecology and Environment 2(4): 236–249. http://doi.org/10.1007/s40974-017-0064-9
Kurth, V.J., D’Amato, A.W., Palik, B.J., Bradford, J.B., 2014: Fifteen-Year Patterns of Soil Carbon and Nitrogen Following Biomass Harvesting. Soil Sci Soc Am J 78(2): 624–633. http://doi.org/10.2136/sssaj2013.08.0360
Lukina, N.V., Tikhonova, E.V., Danilova, M.A., Bakhmet, O.N., Kryshen, A.M., Tebenkova, D.N., Kuznetsova, A.I., Smirnov, V.E., Braslavskaya, T.Y., Gornov, A.V., Shashkov, M.P., Knyazeva, S.V., Kataev, A.D., Isaeva, L.G., Zukert, N.V., 2019: Associations between Forest Vegetation and the Fertility of Soil Organic Horizons in Northwestern Russia. Forest Ecosystems 6(1): 1–19. http://doi.org/10.1186/s40663-019-0190-2
Marchi, E., Picchio, R., Mederski, P.S., Vusić, D., Perugini, M., Venanzi, R., 2016: Impact of Silvicultural Treatment and Forest Operation on Soil and Regeneration in Mediterranean Turkey Oak (Quercus cerris L.) Coppice with Standards. Ecol Eng 95: 475–484. http://doi.org/10.1016/j.ecoleng.2016.06.084
Marshall, V.G., 2000: Impacts of Forest Harvesting on Biological Processes in Northern Forest Soils. For Ecol Manage 133(1): 43–60. http://doi.org/10.1016/S0378-1127(99)00297-2
Marty, C., Houle, D., Gagnon, C., Courchesne, F., 2017: The Relationships of Soil Total Nitrogen Concentrations, Pools and C:N Ratios with Climate, Vegetation Types and Nitrate Deposition in Temperate and Boreal Forests of Eastern Canada. Catena 152: 163–172. http://doi.org/10.1016/j.catena.2017.01.014
McFero Grace, J., Skaggs, R.W., Cassel, D.K., 2006: Soil Physical Changes Associated with Forest Harvesting Operations on an Organic Soil. Soil Sci Soc Am J 70(2): 503–509. http://doi.org/10.2136/sssaj2005.0154
Miller, R.O., Kissel, D.E., 2010: Comparison of Soil Ph Methods on Soils of North America. Soil Sci Soc Am J 74(1): 310–316. http://doi.org/10.2136/sssaj2008.0047
Ministry of Agriculture. 1992: First National Forest Census. Ministry of Agriculture: Athens, Greece, 134 p.
Morris, S.J., Boerner, R.E.J., 1998: Interactive Influences of Silvicultural Management and Soil Chemistry Upon Soil Microbial Abundance and Nitrogen Mineralization. For Ecol Manage 103(2): 129–139. http://doi.org/10.1016/S0378-1127(97)00183-7
Mroz, G.D., Jurgensen, M.F., Frederick, D.J., 1985: Soil Nutrient Changes Following Whole Tree Harvesting on Three Northern Hardwood Sites. Soil Sci Soc Am J 49(6): 1552–1557. http://doi.org/10.2136/sssaj1985.03615995004900060044x
Myers, D.T., Rediske, R.R., McNair, J.N., 2019: Measuring Streambank Erosion: A Comparison of Erosion Pins, Total Station, and Terrestrial Laser Scanner. Water 11(9): 1846. https://doi.org/10.3390/w11091846
Naghdi, R., Solgi, A., Ilstedt, U., 2016a: Soil Chemical and Physical Properties after Skidding by Rubber-Tired Skidder in Hyrcanian Forest, Iran. Geoderma 265: 12–18. http://doi.org/https://doi.org/10.1016/j.geoderma.2015.11.009
Naghdi, R., Solgi, A., Labelle, E.R., Zenner, E.K., 2016b: Influence of Ground-Based Skidding on Physical and Chemical Properties of Forest Soils and Their Effects on Maple Seedling Growth. European Journal of Forest Research 135(5): 949–962. http://doi.org/10.1007/s10342-016-0986-3
Naghdi, R., Solgi, A., Zenner, E.K., 2015: Soil Disturbance Caused by Different Skidding Methods in Mountainous Forests of Northern Iran. International Journal of Forest Engineering 26(3): 212–224. http://doi.org/10.1080/14942119.2015.1099814
Nikooy, M., Rashidi, R., Kocheki, G., 2010: Residual Trees Injury Assessment after Selective Cutting in Broadleaf Forest in Shafaroud. Caspian Journal of Environmental Sciences 8(2): 173–179.
Nikooy, M., Tavankar, F., Naghdi, R., Ghorbani, A., Jourgholami, M., Picchio, R., 2020: Soil Impacts and Residual Stand Damage from Thinning Operations. International Journal of Forest Engineering 31(2): 126–137. http://doi.org/10.1080/14942119.2020.1744954
Nordén, B., Ryberg, M., Götmark, F., Olausson, B., 2004: Relative Importance of Coarse and Fine Woody Debris for the Diversity of Wood-Inhabiting Fungi in Temperate Broadleaf Forests. Biol Conserv 117(1): 1–10. http://doi.org/10.1016/S0006-3207(03)00235-0
Palviainen, M., Finér, L., Kurka, A.M., Mannerkoski, H., Piirainen, S., Starr, M., 2004: Decomposition and Nutrient Release from Logging Residues after Clear-Cutting of Mixed Boreal Forest. Plant Soil 263(1): 53–67. http://doi.org/10.1023/B:PLSO.0000047718.34805.fb
Papaioannou, A., 2013: Assessment of the Empirical Management Method of Coppice Chestnut (Castanea Sativa Mill.) Forests Practiced by the Monks and Its Effect on the Availability of Forest Soil Resources in Mount Athos, Greece. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 41(1): 317–325. http://doi.org/10.15835/nbha4118288
Papaioannou, A.G., 2015: Ecological and Soil Conditions of Black Pine (Pinus Nigra Arn.) Stands in the Area of the Russian Monastery at Mount Athos. Russ J Ecol 46(5): 438–443. http://doi.org/10.1134/s1067413615050161
Papamichos, N., Alifragis, D., 1990: Forest Soil Fertility. Dedousis: Thessaloniki. 15 p.
Paudel, E., Dossa, G.G.O., De BleCourt, M., Beckschafer, P., Xu, J., Harrison, R.D., 2015: Quantifying the Factors Affecting Leaf Litter Decomposition across a Tropical Forest Disturbance Gradient. Ecosphere 6(12): 1–20. http://doi.org/10.1890/ES15-00112.1
Pereira, P., Brevik, E.C., Muñoz-Rojas, M., Miller, B.A., Smetanova, A., Depellegrin, D., Misiune, I., Novara, A., Cerdà, A., 2017: Chapter 2 – Soil Mapping and Processes Modeling for Sustainable Land Management. In: Soil Mapping and Process Modeling for Sustainable Land Use Management; Pereira, P., Brevik, E.C., Muñoz-Rojas, M., Miller, B.A. Eds; Elsevier.
Powers, R.F., Andrew Scott, D., Sanchez, F.G., Voldseth, R.A., Page-Dumroese, D., Elioff, J.D., Stone, D.M., 2005: The North American Long-Term Soil Productivity Experiment: Findings from the First Decade of Research. For Ecol Manage 220(1): 31–50. http://doi.org/https://doi.org/10.1016/j.foreco.2005.08.003
Prescott, C.E., 1997: Effects of Clearcutting and Alternative Silvicultural Systems on Rates of Decomposition and Nitrogen Mineralization in a Coastal Montane Coniferous Forest. For Ecol Manage 95(3): 253–260. http://doi.org/10.1016/S0378-1127(97)00027-3
Pritchett, W.L., 1979: Properties and Management of Forest Soils. John Wiley & Sons New York, USA, 500 p.
R Core Team, 2018: R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. https://www.R-project.org
Remezov, N.P., Pogrebnyak, P.S., 1969: Forest Soil Science. Israel Program for Scientific Translations: Jerusalem, 261 p.
Ross, D.S., Bailey, S.W., Lawrence, G.B., Shanley, J.B., Fredriksen, G., Jamison, A.E., Brousseau, P.A., 2011: Near-Surface Soil Carbon, Carbon/Nitrogen Ratio, and Tree Species Are Tightly Linked across Northeastern United States Watersheds. For Sci 57(6): 460–469. http://doi.org/10.1093/forestscience/57.6.460
Ryan, D.F., Huntington, T.G., Wayne Martin, C., 1992: Redistribution of Soil Nitrogen, Carbon and Organic Matter by Mechanical Disturbance During Whole-Tree Harvesting in Northern Hardwoods. For Ecol Manage 49(1): 87–99. http://doi.org/10.1016/0378-1127(92)90162-3
Salmon, S., 2018: Changes in Humus Forms, Soil Invertebrate Communities and Soil Functioning with Forest Dynamics. Appl Soil Ecol 123: 345–354. http://doi.org/10.1016/j.apsoil.2017.04.010
Schmitz, M.a.F., Atauri, J.A., de Pablo, C.L., de Agar, P.M.n., Rescia, A.J., Pineda, F.D., 1998: Changes in Land Use in Northern Spain: Effects of Forestry Management on Soil Conservation. For Ecol Manage 109(1): 137–150. http://doi.org/10.1016/S0378-1127(98)00241-2
Schoenholtz, S.H., Miegroet, H.V., Burger, J.A., 2000: A Review of Chemical and Physical Properties as Indicators of Forest Soil Quality: Challenges and Opportunities. Forest Ecology and Management 138(1–3): 335–356. http://doi.org/10.1016/S0378-1127(00)00423-0
Serengil, Y., Gökbulak, F., Özhan, S., Hızal, A., Şengönül, K., Nihat Balcı, A., Özyuvacı, N., 2007: Hydrological Impacts of a Slight Thinning Treatment in a Deciduous Forest Ecosystem in Turkey. Journal of Hydrology 333(2): 569–577. http://doi.org/10.1016/j.jhydrol.2006.10.017
Shrestha, S.P., Lanford, B.L., Rummer, R., Dubois, M., 2008: Soil Disturbances from Horse/Mule Logging Operations Coupled with Machines in the Southern United States. International Journal of Forest Engineering 19(1): 17–23. http://doi.org/10.1080/14942119.2008.10702555
Slesak, R.A., Palik, B.J., D'Amato, A.W., Kurth, V.J., 2017: Changes in Soil Physical and Chemical Properties Following Organic Matter Removal and Compaction: 20-Year Response of the Aspen Lake-States Long Term Soil Productivity Installations. For Ecol Manage 392: 68-77. http://doi.org/10.1016/j.foreco.2017.03.005
Solgi, A., Naghdi, R., Labelle, E.R., Tsioras, P.A., Nikooy, M., 2016: Effect of Varying Machine Ground Pressure and Traffic Frequency on the Physical Properties of Clay Loam Soils Located in Mountainous Forests. International Journal of Forest Engineering 27(3): 161–168. http://doi.org/10.1080/14942119.2016.1226673
Spinelli, R., Lombardini, C., Magagnotti, N., 2014: The Effect of Mechanization Level and Harvesting System on the Thinning Cost of Mediterranean Softwood Plantations. Silva Fenn 48(1): 1003. http://doi.org/10.14214/sf.1003
Stevenson, F.J., 1982: Nitrogen-Organic Forms. In: Methods of Soil Analysis Part 2 Chemical and Microbiological Properties, American Society of Agronomy Inc., Soil Science Society of America Inc., 625–641. http://doi.org/10.2134/agronmonogr9.2.2ed: 625-641.
Swank, W.T., Webster, J.R., 2014: Long-Term Response of a Forest Watershed Ecosystem: Clearcutting in the Southern Appalachians. Oxford University Press New York, New York, USA, 272 p.
Tavankar, F., Bonyad, A.E., Nikooy, M., Picchio, R., Venanzi, R., Calienno, L., 2017: Damages to Soil and Tree Species by Cable-Skidding in Caspian Forests of Iran. Forest Systems 26(1): 11. http://doi.org/10.5424/fs/2017261-09100
Taxiarhis University Forest, 1991: Forest Management Plan. Aristotle University of Thessaloniki: Taxiarhis, Greece, 71 p.
Tsioras, P.A., 2010: Perspectives of the Forest Workers in Greece. iForest 3(5): 118–123. http://doi.org/10.3832/ifor0547-003
Tsioras, P.A., 2012: Status and Job Satisfaction of Greek Forest Workers. Small-scale Forestry 11(1): 1–14. http://doi.org/10.1007/s11842-011-9164-0
Tsioras, P.A., Liamas, D.K., 2015: Residual Tree Damage Along Skidding Trails in Beech Stands in Greece. Journal of Forestry Research 26(2): 523–531. http://doi.org/10.1007/s11676-015-0056-6
Tutin, T.G., Burges, N.A., Chater, A.O., Edmonson, J.R., Heywood, V.H., M., M.D., Valentine, D.H., Walters, S.M., Webb, D.A., 1993: Flora Europaea 2nd ed. 5: 72–76. Cambridge University Press, Cambridge, Great Britain.
Venanzi, R., Picchio, R., Piovesan, G., 2016: Silvicultural and Logging Impact on Soil Characteristics in Chestnut (Castanea Sativa Mill.) Mediterranean Coppice. Ecol Eng 92: 82–89. http://doi.org/10.1016/j.ecoleng.2016.03.034
Vitousek, P.M., Matson, P.A., 1985: Intensive Harvesting and Site Preparation Decrease Soil Nitrogen Availability in Young Plantations. South J Appl For 9(2): 120–125. https://doi.org/10.1093/sjaf/9.2.120
Wallace, E.S., Freedman, B., 1986: Forest Floor Dynamics in a Chronosequence of Hardwood Stands in Central Nova Scotia. Can J For Res 16(2): 293–302. http://doi.org/10.1139/x86-050
Wang, L., 1997: Assessment of Animal Skidding and Ground Machine Skidding under Mountain Conditions. Journal of Forest Engineering 8(2): 57–64.
Wang, L., 1999: Environmentally Sound Timber Extraction Techniques for Small Tree Harvesting. ASAE Annual International Meeting. 18–21 July, Toronto, Ontario, Canada. Paper No. 995053, 7 p.
World Resources Institute. Available online: https://www.wri.org/blog/2019/04/world-lost-belgium-sized-area-primary-rainforests-last-year.
Worrell, R., Hampson, A., 1997: The Influence of Some Forest Operations on the Sustainable Management of Forest Soils – a Review. Forestry 70(1): 61–85. https://doi.org/10.1093/forestry/70.1.61
Yamakura, T., Sahunalu, P., 1990: Soil Carbon/Nitrogen Ratio as a Site Quality Index for Some South-East Asian Forests. J Trop Ecol 6(3): 371–377.
Yanai, R.D., 1998: The Effect of Whole-Tree Harvest on Phosphorus Cycling in a Northern Hardwood Forest. For Ecol Manage 104(1–3): 281–295. http://doi.org/10.1016/s0378-1127(97)00256-9
Yanai, R.D., Arthur, M.A., Siccama, T.G., Federer, C.A., 2000: Challenges of Measuring Forest Floor Organic Matter Dynamics:: Repeated Measures from a Chronosequence. For Ecol Manage 138(1): 273–283. http://doi.org/10.1016/S0378-1127(00)00402-3
Zar, J.H., 1999: Biostatistical Analysis. 4th ed.; Prentice Hall: Upper Saddle River, New Jersey, USA.
Zenner, E.K., Fauskee, J.T., Berger, A.L., Puettmann, K.J., 2007: Impacts of Skidding Traffic Intensity on Soil Disturbance, Soil Recovery, and Aspen Regeneration in North Central Minnesota. North J Appl For 24(3): 177–183. http://doi.org/10.1093/njaf/24.3.177
© 2021 by the authors. Submitted for possible open access publication under the
terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Authors’ addresses:
Harisios P. Ganatsios, PhD
e-mail: cganats@for.auth.gr
Aristotle University of Thessaloniki
Faculty of Forestry and Natural Environment
Laboratory of Mountainous Water Management and Control
POB 225, GR-541 24 Thessaloniki
GREECE
Petros A. Tsioras, PhD*
e-mail: ptsioras@for.auth.gr
Aristotle University of Thessaloniki
Faculty of Forestry and Natural Environment
Laboratory of Forest Utilization
POB 227, GR-541 24 Thessaloniki
GREECE
Assoc. prof. Athanasios G. Papaioannou, PhD
e-mail: apapaioa@for.auth.gr
Aristotle University of Thessaloniki
Faculty of Forestry and Natural Environment
Laboratory of Forest Soil Science
POB 271, GR-541 24 Thessaloniki
GREECE
Prof. Charles R. Blinn, PhD
e-mail: cblinn@umn.edu
University of Minnesota
Department of Forest Resources
1530 Cleveland Avenue North
St. Paul, MN 55108
UNITED STATES
* Corresponding author
Received: 1 July 2020
Accepted: 30 December 2020
Original scientific paper

