Variability of Morpho-Anatomical Characteristics of Different Willow Clones Contaminated with Heavy Metals
doi: 10.5552/crojfe.2024.2289
volume: 45, issue: 2
pp: 12
- Author(s):
-
- Jokanović Dušan
- Urošević Jelena
- Stojnić Srđan
- Nikolić Jokanović Vesna
- Stanković Dragica
- Ištok Iva
- Article category:
- Original scientific paper
- Keywords:
- Salix clones, polluted site, control site, morpho-anatomical characteristics
Abstract
HTML
In this paper, the variability of morphological (stem height, stem basal diameter, proportion of pith, wood and bark) and wood anatomical characteristics (fiber length, fiber diameter, fiber lumen diamater, double cell-wall thickness, vessel diameter, wood rays width and height) of three Salix alba clones (B-44, 347 and NS 73/6) and one Salix viminalis clone both in the control plot and in the site contaminated with a mixture of heavy metals (As, Cd, Cr, Cu, Ni, Pb) was investigated. The observed results showed that individuals of all four clones had significantly higher average values of stem height and stem basal diameter at the control plot compared to the polluted site. As for the proportion of pith, bark and wood, heavy metals caused an increase in the share of pith and a decrease in the share of bark and wood in all clones with the exception of clone NS 73/6. The analysis of wood fiber dimensions showed that the values of all parameters were higher at the control site with the exception of fiber lumen diameter where higher values were observed for clones B-44 and NS 73/6 at the polluted site. Higher values of vessel diameter were recorded for all clones at the control plot, while wood rays width of all individuals was greater at the contaminated site. Regarding the wood rays height, only Salix viminalis showed higher value at the polluted site. These results confirmed that pollution-induced heavy metal stress significantly altered the morphological and wood anatomical characteristics of all researched clones and that it may affect their utility properties.
Variability of Morpho-Anatomical Characteristics of Different Willow Clones Contaminated with Heavy Metals
Dušan Jokanović, Jelena Urošević, Srđan Stojnić, Vesna Nikolić Jokanović, Dragica Stanković, Iva Ištok
Abstract
In this paper, the variability of morphological (stem height, stem basal diameter, proportion of pith, wood and bark) and wood anatomical characteristics (fiber length, fiber diameter, fiber lumen diamater, double cell-wall thickness, vessel diameter, wood rays width and height) of three Salix alba clones (B-44, 347 and NS 73/6) and one Salix viminalis clone both in the control plot and in the site contaminated with a mixture of heavy metals (As, Cd, Cr, Cu, Ni, Pb) was investigated. The observed results showed that individuals of all four clones had significantly higher average values of stem height and stem basal diameter at the control plot compared to the polluted site. As for the proportion of pith, bark and wood, heavy metals caused an increase in the share of pith and a decrease in the share of bark and wood in all clones with the exception of clone NS 73/6. The analysis of wood fiber dimensions showed that the values of all parameters were higher at the control site with the exception of fiber lumen diameter where higher values were observed for clones B-44 and NS 73/6 at the polluted site. Higher values of vessel diameter were recorded for all clones at the control plot, while wood rays width of all individuals was greater at the contaminated site. Regarding the wood rays height, only Salix viminalis showed higher value at the polluted site. These results confirmed that pollution-induced heavy metal stress significantly altered the morphological and wood anatomical characteristics of all researched clones and that it may affect their utility properties.
Keywords: Salix clones, polluted site, control site, morpho-anatomical characteristics
1. Introduction
Heavy metals have a great impact on morpho-anatomical plant characteristics (Nikolić 2009). However, to understand the way they impact plants, it is necessary to know how heavy metals reach the plant organism, as well as the process of their uptake and accumulation, and finally how they are transported from the root to the aboveground parts (Arsenov 2018). The greatest quantity of heavy metals is located in the soil, but plant uptake of these substances is directly dependent on the content of available forms of heavy metals in the soil (Chaney et al. 2007). Soil remediation is mainly related to the presence of heavy metals in citosol in available form to plants and includes a few stages: heavy metal transition from solid to another state; transport of the released pollutant; its attachment to the root; passage through the cell membrane and entry in metabolic processes in the cell (Kim et al. 2015).
Root is the main organ in charge of heavy metals absorption and accumulation, while the cell wall is the central part of heavy metals linkage (Nikolić 2009). After reaching the root, heavy metals can be immobilized and kept at the root level or they can be transported via the xylem sap to the aerial parts of the plants where they are mainly deposited in the vacuole (Seregin and Kozhevnikova 2008). Willows (Salix spp.) are hyperaccumulators due to tolerance to high concentrations of heavy metals, so these species developed detoxification mechanisms (Arsenov 2018). Hyperaccumulation of metals is actually an ecophysiological adaptation of plants to grow unhindered on contaminated soil.
Many studies confirmed different environmental changes caused by different factors and their effect on the anatomical structure of vascular plants (Iqbal et al. 2010, Rajput et al. 2008, Rajput and Rao 2005, Safdari et al. 2012). Ahmad et al. (2005) investigated the influence of higher cadmium and lead content by Trigonella foenum-graecum L. and, as a consequence, they found decreased dimensions of wood vessels and fibers. Wali et al. (2007) established similar tendencies by Calendula officinalis L. under the influence of SO2 in laboratory conditions. Some authors (Carlquist 1977, De Melo et al. 2018) agreed on challenges in determining specific stress factors in the field-experiments, but they also concluded that the human factor is the most responsible for environmental stresses affecting the quality of wood. Gupta and Iqbal (2005) emphasised that the effects of environmental stress on the quality of wood mainly depend on tree species.
The presence of willows can be benificial for the environment for many reasons – they generally have a significantly positive influence on biodiversity (Baum et al. 2012) and their early flowering and spreading of pollen greatly accelerates the pollination process (Christersson 2013). Willows also reduce soil erosion, they are not at all demanding on the habitat, and hardly deplete the soil in terms of nutrient consumption. It is also a very beneficial species due to its ability to clean waste water from high toxicity. It is a well-known fact that willows can be used for accumulating Cd from the soil, which contributes a lot to reduced concentrations of this very toxic element (Mirck et al. 2005).
Willow is widely used for so-called green technology purposes, as it cleans soils contaminated with high concentrations of heavy metals and contributes very positively to the envoronment as a whole (Pulford et al. 2002). Bearing in mind that willows are characterized by short rotation period, they are often planted for biomass and energy production (Pulford et al. 2002). Some authors (Alloway 1995, Ross 1994) related high soil toxicity with different human influences such as mining activity, heavy industry, overpopulated urban areas, etc. One of the main reasons for the accumulation of heavy metals in the biosphere is that they are non-degradable elements, so they can not be destroyed, but only transformed into less toxic forms or can be deposited in chemically inactive forms (Arsenov 2018).
The aim of this paper was to investigate the variability between morphological (stem height, stem basal diameter, proportion of wood, bark and pith) and wood anatomical (wood rays height, wood rays width, vessel diameter, fiber length, fiber width, fiber lumen diameter, double cell-wall thickness) characteristics of four willow clones cultivated under optimal conditions and at the site contaminated with different heavy metals (As, Cd, Cr, Cu, Ni, Pb) in order to evaluate the heavy-metals soil pollution effect on the morpho-anatomical structure of the studied clones.
2. Materials and Methods
2.1 Experimental Set-Up
For the purpose of this research, four willow clones were selected – one is Salix viminalis and three clones belong to the Salix alba: cl. B-44, cl. 347 and cl. NS 73/6. The clones were selected while they were produced and available in the nursery of PE »Vojvodinašume«. Willow was selected as a species that is a hyperaccumulator and has a great ability to absorb and accumulate heavy metals. Cuttings were directly planted in the nursery of the University of Blegrade, Faculty of Forestry (Serbia), which represents a control area, on which there is no contamination, nor the influence of the human factor. Cuttings were also planted in contaminated soil collected from the area of the Mining Basin »Kolubara«, with the aim to establish how high toxicity affects the characteristics of morpho-anatomical wood of different willow clones. The soil was dug with an excavator from different depths and then homogenized, i.e. mixed and distributed in polyethylene bags with a volume of 10 liters. Bags filled with this substrate were transported to the nursery of the Faculty of Forestry in Belgrade where three cuttings of each clone were planted in each bag. For the analyses of the morpho-anatomical characteristics, one bag for each clone was selected, which in total amounts to 12 cuttings from the contaminated site. The same number of samples was analyzed from the control plot, so the total number of analyzed individuals was 24.
Stem cuttings were cut when second growing period was finished, because during two years a significant amount of heavy metals was deposited both in the soil and stem. The willow cuttings were soaked in the fungicide diacopper-chloride-trihydroxide before pricking. Additional treatment of soil from the Mining Basin »Kolubara« was carried out with a mixture consisting of all six heavy metals at the beginning of the first and second vegetation period. For the purpose of additional contamination, aqueous solutions of salts of heavy metals were used, namely: Cd (NO3)2, CuSO4*5H2O, K2Cr2O7, HAsNa2O4*7H2O, NiCl2*6H2O and PbNO3 in concentrations of 10-3 mol/dm3.
2.2 Determination of Physical and Chemical Properties of Soil
Soil analyses were conducted in the Laboratory of Forestry Institute in Belgrade and included:
the content of total organic matter (humus) determined by wet combustion in a mixture of potassium dichromate and sulfuric acid according to the Tjurin method as modified by Simakov
the total nitrogen content was determined by digesting the soil sample in concentrated sulfuric acid with the presence of a catalyst and distilling NH3 using the Kjeldach method
active and substitution acidity were determined conductometrically on a pH meter
the content of free ground alkaline carbonates was determined by the volumetric method according to the effect of hydrochloric acid solution on the soil and the measurement of the volume of released carbon dioxide
the textural composition of the soil was determined by the sedimentation method using sodium pyrophosphate as a peptizing agent and the textural class was determined using the Ferre triangle.
As for the substrate at the control site (Table 1), the most represented fraction is fine sand, followed by clay and powder. Infiltration and filtration of water into the soil at the control site is slower than in the case with ideal substrates for nurseries (clay composition), however, there are suitable conditions for drainage and the entry of air into the drainage pores. The most abundant fraction in the texture composition of the contaminated substrate is the clay fraction (particles smaller than 0.002 mm) followed by powder. Due to the heavier texture composition, the substrate at the contaminated site is less permeable to water and less aerated than the substrate at the control plot.
Table 1 Soil texture composition at control and contaminated site
|
Texture composition |
Soil type |
|||||
|
Substrate at control site |
Substrate at contaminated site |
|||||
|
Average |
Standard deviation |
Mean error of the arithmetic mean |
Average |
Standard deviation |
Mean error of the arithmetic mean |
|
|
Coarse sand, % |
8.37 |
±0.60 |
1.4583 |
11.72 |
1.4865 |
±0.61 |
|
Small sand, % |
33.75 |
±0.36 |
0.8781 |
26.52 |
1.6798 |
±0.69 |
|
Powder, % |
27.17 |
±0.57 |
1.3995 |
28.00 |
1.9026 |
±0.78 |
|
Clay, % |
30.72 |
±0.20 |
0.4916 |
33.77 |
1.1201 |
±0.46 |
|
Total sand, % |
42.12 |
±0.57 |
1.3949 |
38.23 |
2.5057 |
±1.02 |
|
Total clay, % |
57.88 |
±0.57 |
1.3949 |
61.77 |
2.5057 |
±1.02 |
The active acidity of the soil solution of the substrate at the control site was 7.89±0.01 pH units, while at the contaminated site it was 7.78±0.03 (Table 2). Substitution acidity of the substrate at the control site was 7.11±0.02 pH units and a bit smaller at the contaminated site (Table 2). The slightly to moderately alkaline reaction of the soil solution at the control site was a consequence of the presence of free carbonates. According to the content of free carbonates, the substrate at the control site belongs to low carbonate soils with an average content of 5.23±0.08% (Table 2). The content of total humus and organic matter was 2.69±0.04%, which classifies the substrate at the control site as weakly humus soils. The soil was very well provided with phosphorus, which is easily accessible to plants, and also with potassium. The active acidity of the soil solution of the substrate at the contaminated site was 7.78±0.03 pH units, which classifies this substrate as a weakly alkaline soil, while the substitution acidity was 7.05±0.01 pH units. Despite the slightly alkaline reaction of the soil solution, no large amounts of free carbonates were found in the substrate samples at the contaminated site. In all analyzed samples of the substrate at the contaminated site, the amount of carbonate was less than 1%, which classifies this substrate as non-carbonate soils. Such a low content of carbonates, at a fairly high pH value of the soil, indicated that the alkaline reaction did not come from carbonates of ground-alkaline elements (Ca and Mg), but from carbonates of alkaline elements (Na and K). The content of humus in the substrate at the contaminated site was 5.03±0.16% (Table 2). This indicates that the substrate at the contaminated site, according to the humus content, was at the transition between quite humus and very humus. Total nitrogen content was highly variable at the contaminated site, and the C/N ratio was wide. This means that the processes of mineralization of nitrogen organic forms are slowed down, that most of the mineral forms of nitrogen released from organic matter are used by saprophytic microorganisms, and that only a small part remains for plants. The average content of phosphorus easily accessible to plants in the substrate at the contaminated site was 13.89±0.74 mg/100 g of soil, which classifies this substrate as mediumly provided with phosphorus. According to the content of potassium forms easily accessible to plants (19.05±0.50 mg/100 g of soil), the substrate at the contaminated site was at the transition between medium and well provided.
Table 2 Soil chemical characteristics
|
Soil chemical characteristics |
Soil type |
|||||||
|
Substrate at control site |
Substrate at contaminated site |
|||||||
|
Average |
Standard deviation |
Mean error of arithmetic mean |
Average |
Standard deviation |
Mean error of arithmetic mean |
|||
|
pH |
H2O |
7.89 |
0.0242 |
±0.01 |
7.78 |
0.0677 |
0.03 |
|
|
KCl |
7.11 |
0.0412 |
±0.02 |
7.05 |
0.0160 |
0.01 |
||
|
CaCO3 |
% |
5.23 |
0.1984 |
±0.08 |
0.53 |
0.2792 |
0.11 |
|
|
Total |
Humus |
% |
2.69 |
0.1014 |
±0.04 |
5.03 |
0.3909 |
0.16 |
|
N |
% |
0.17 |
0.0575 |
±0.02 |
0.16 |
0.0342 |
0.01 |
|
|
C/N |
9.83 |
3.408 |
±1.39 |
18.90 |
3.3994 |
1.39 |
||
|
Accessible |
P2O5 |
mg/100g |
25.67 |
1.6898 |
±0.69 |
13.89 |
1.8047 |
0.74 |
|
K2O |
mg/100g |
26.20 |
1.1713 |
±0.48 |
19.05 |
1.2276 |
0.50 |
|
2.3 Morphological Analyses
At the end of the second growing season, cuttings of 4 willow clones were harvested at 3 cm above the ground level both at the control and contaminated site and three individuals were collected from each clone. In order to avoid the influence of surrounding trees, samples were taken at the distance of 2 m between each other. All cuttings were two years old. A total of 24 cuttings for all willow clones (3 at control and 3 at the contaminated site for each clone) were cut and all cuttings were produced from the dormant shoots. In order for the samples to reach the laboratory in an undamaged state, immediately after cutting, they were wrapped in wet paper and put in plastic bags to avoid the loss of water.
Five morphological characteristics were investigated for each cutting: stem height, stem basal diameter, pith proportion (%), xylem proportion (%) and bark proportion (%). The stem height was measured using a metric folding ruler from the ground level up to the top of the stem. The stem basal diameter was determined at the base of the stem (near the ground level), and it was measured together with the bark using a digital calliper. After the stem diameter was determined, the bark was removed and the remaining diameter, including both xylem and pith, was calculated. The diameter of the central stem part (pith) was measured in two different directions standing at an angle of 180° between each other. In order to establish the participation of three different segments (pith, bark and xylem), it was necessary to calculate the total stem diameter and then determine the percentage of each of these parts in relation to the total diameter (Özden and Ennos 2018). In case of such young cuttings, percentage proportion of each of these parts is much more representative information than its size expressed in appropriate units.
2.4 Wood Anatomical Analyses
Performing anatomical analyses was related to preparing cross-sections from trees which were then cut into small pieces (around 1 cm length). In order to obtain thin sections, it was necessary to soften the material in boiling water, and then to keep it in the mixture of water, glycerol and ethanol in the same proportions (Yaltirik 1971). The samples were then cut using sliding microtome in both cross and tangential sections, 20–25 microns thick. As for measuring certain elements, vessel diameter (VD) was determined from the cross-section, while wood ray width (WRW) and wood ray height (WRH) were calculated from the tangential section. From each of the cross-sections, radial dimensions of 60 vessels were measured. Vessel diameter (VD) was calculated in two transverse directions, and then the arithmetic mean was taken as a weight. Width and height of 60 wood rays per tangential section were determined. Completely undamaged wood rays were selected for determining their width and height that were expressed in microns, which is more suitable than in number of parenchyma cells. As for determining the fiber cell anatomical dimensions, it was necessary to treat prepared small strips using Franklin's (1945) method of maceration. Maceration is a process of chemical decomposition of wood in order to disintegrate the intercellular spaces, and it gives the possibility of measuring the dimensions of wood fibers. The material, chopped into very small pieces, was placed in glass test tubes with a volume of 50 cm3, in which glacial acetic acid H2O2 was previously mixed in an equal volume ratio. The test tube was then shaken to mix the ingredients, and the prepared macerate was thermally treated. The test tubes were closed from the top with a cork stopper so that the prepared material would not evaporate, and then placed in a drying oven at a temperature of 65° where they were kept for 24 hours. At the end of this process, the wood was decomposed to the desired extent, and then the obtained material was washed with a 50% alcohol solution and placed on a glass slide, where undamaged fibers were separated with special needles. For each anatomical characterization of wood fibers (fibre length (FL), fibre diameter (FD), fibre lumen diameter (FLD) and double-cell wall thickness (DCWT)), 40 measurements were done (IAWA 1989). All cell measurements were done using a light microscope Boeco and digital photographs were obtained by specialized Image Analysis Software (Version 3.4.0. 2016).
2.5 Statistical Analyses
Statistical analyses were carried out using Statistica 12 (StatSoft Inc. 2012) software. Normality of data distribution was assessed using Shapiro-Wilk's test. Two-factorial analysis of variance (ANOVA) and Tukey's HSD (honestly significant difference) test were applied to test significance levels (p<0.05) for the effects of clone, treatment and their interactions. Relationships between the studied wood anatomical characteristics and stem basal diameter and stem height were evaluated by Pearson's correlation coefficient in control and heavy-metal treatments, respectively.
3. Results and Discussion
According to the results of the F-test of two-way ANOVA, variation of the studied characteristics, except WRW and FLD, was significantly influenced by both factors (clone and treatment). Indeed, no significant effect of »clone« and »treatment« were found for WRW and FLD, respectively (Table 3). Moreover, two-factorial ANOVA revealed a significant effect of »clone by treatment« interaction for all examined parameters, except FD.
Table 3 Results of F-test on examined wood anatomical and biometrical characteristics in Salix viminalis and Salix alba clones
|
Source |
FL |
FD |
FLD |
DCWT |
VD |
WRW |
WRH |
SBD |
SH |
|
Clone A |
39.84*** |
10.62*** |
7.07*** |
6.82*** |
136.30*** |
0.80ns |
28.93*** |
597.71*** |
3739.72*** |
|
Treatment B |
6.93** |
9.37** |
1.02ns |
34.78*** |
107.32*** |
33.06*** |
19.01*** |
14234.90*** |
34029.51*** |
|
Interaction AxB |
8.09*** |
2.45ns |
4.24** |
4.59** |
7.04*** |
3.48* |
9.06*** |
406.55*** |
1980.14*** |
|
ns – non-significant; (*) p<0.05; (**) p<0.01; (***) p<0.001 |
|||||||||
The individuals of S. alba cl. NS 73/6 showed the most stable performance in both treatments of all tested clones, having the highest mean values of FL and VD, and belonging to the first homogeneous group in terms of FD, FLD and WRH (Table 4). In addition, the highest mean value of DCWT was also observed in S. alba cl. NS 73/6 in the control site, while the other clones showed no statistically significant differences for this trait regardless of the treatment. In contrast, clone B-44 was characterized by low values of FL, FD, FLD in both treatments, whereas clone 347 plants had the lowest values regarding WRH in both treatments, and FD, FLD, DCWT and VD (together with S. viminalis) in treatment with heavy metals.
The analysis of the results for FL showed that all clones have longer fibers at the control site, except Salix viminalis, where longer fibers were determined at the contaminated site (Table 4). When comparing the mean values of the length of wood fibers at the control site, the longest fibers are present in clone NS 73/6 (0.734 mm), and the shortest in clone B-44 (0.573 mm). Regarding the FD, in all clones, higher values were recorded at the control site with the exception of clone B-44 (Table 4), while comparing the values of this parameter in control conditions showed that the widest fibers are present in Salix viminalis clone (19.88 µm). As for fiber lumen diameter (FLD), higher values were determined at the contaminated site in clones B-44 and NS 73/6, while clones Salix viminalis and 347 had greater values at the control site, and when comparing mean values of this parameter at the control site, the greatest fiber lumen diameter was recorded in Salix viminalis (13.97 µm). The last analyzed characteristic related to wood fibers – DCWT showed higher values at the control site for all investigated clones, while the greatest DCWT at the control site was recorded in clone NS 73/6 – 7.05 µm. Based on the results for the dimensions of different wood fiber parameters, it can be concluded that all dimensions are larger at the control site apart from the fiber lumen diameter where the ratio between the clones is equal. Mulenga et al. (2022) investigated Brachystegia longifolia trees and found that environmental stress caused by high heavy metals concentrations significantly affects decreased dimensions of all wood fiber elements, which is in accordance with our results, although differences between these values at the control and contaminated site by willow clones are much lower. It can be assumed that more favorable soil C/N ratio at the control plot positively affects the height and radial increment of the plants (Table 2). Namely, if the C/N ratio is over 10, a large amount of available nitrogen is used by microorganisms, thus reducing soil fertility and productivity (Antić et al. 1982). It is evident that the dynamics of radial and height reducing growth of willow cuttings is directly proportional to the dimensions of wood fibers.
Table 4 Mean values and standard deviation of wood anatomical characteristics investigated under two treatments. Differences between values of the same characteristics that are labeled with the same letter are not statistically significant (p>0.05). The parameter acronyms are defined in Material and Methods
|
Clone |
Treatment |
Control |
||||||
|
Salix viminalis |
cl. B-44 |
cl. 347 |
cl. NS 73/6 |
Salix viminalis |
cl. B-44 |
cl. 347 |
cl. NS 73/6 |
|
|
FL |
0.675±0.141abc |
0.513±0.091f |
0.546±0.106ef |
0.699±0.010ab |
0.611±0.107cde |
0.573±0.095def |
0.637±0.092bcd |
0.734±0.131a |
|
FD |
17.48±3.57abc |
19.92±3.57c |
15.55±3.10c |
19.58±5.63ab |
19.88±4.27a |
17.09±3.76bc |
18.01±3.35abc |
19.70±3.33a |
|
FLD |
12.25±3.28abc |
11.77±3.08bc |
10.49±2.75c |
14.30±4.68a |
13.97±4.37ab |
11.65±3.48bc |
12.15±3.42abc |
12.63±3.72abc |
|
DCWT |
5.21±0.82b |
5.19±1.06b |
5.10±1.36b |
5.32±1.70b |
5.94±1.92b |
5.41±1.31b |
5.87±1.11b |
7.05±1.27a |
|
VD |
14.46±3.71d |
22.15±7.23c |
16.91±4.18d |
32.55±6.57b |
23.46±4.99c |
25.35±7.81c |
25.33±6.70c |
36.11±8.33a |
|
WRW |
40.03±13.15a |
36.79±6.84ab |
35.71±9.30abc |
32.51±8.94bcd |
28.01±8.12d |
29.19±8.25cd |
30.81±6.34bcd |
30.60±8.63bcd |
|
WRH |
226.5±43.50b |
224.90±64.60b |
221.80±49.70b |
307.30±96.50a |
225.00±83.90b |
348.50±95.50a |
221.90±79.80b |
355.10±72.30a |
As for vessel diameter (Table 4), for all four clones higher values were recorded at the control compared to the contaminated site, while at the control plot, the largest vessel diameter was observed in clone NS 73/6 (36.11 µm). Based on this, it can be concluded that polluted environment reduces the width of conducting elements in willows. As a result, they gradually narrow the vessels in order to prevent heavy metals transport through the cytoplasmic matrix and thus maintain metabolic processes at an optimal level. Mulenga et al. (2022) investigated Brachystegia longifolia trees and found a reduction of 23% in vessel diameter at the most polluted site, which is compatible with our results where also much narrower vessels were found at the contaminated compared to the control site. Zimmermann (1983) explained that the reduction of vessel dimensions both in height and width is related to ecophysiological adaptation of these species. According to this mechanism, the plant is protected from cell dying by enabling a smooth transport of water and minerals from xylem to phloem and moving of nutrients in a reverse direction (Husen and Iqbal 1999). An increased concentration of heavy metals can greatly impair the maintaining of the transpiration, but hyperaccumulators respond to this by forming an increased number of vessels, thus reducing the negative influence of pollution (Rajput et al. 2008). Metals influence root membrane permeability to water (Przedpelska-Wasowicz and Wierzbicka 2011) that affects vessel dimensions and functioning of conductive tissues. Some authors connected plant responses to heavy metal stress with reduced water content in plant tissues (Poschenrieder and Barcelo 2004). As for the relation between high heavy metal concentration and water content in the plants, hormones can also play a significant role, and it was found that Cd content affects ABA concentrations. However, on the other side, this heavy metal reduces stomatal conductance even in ABA-insesitive mutants (Barcelo et al. 1986, Perfus-Barbeoch et al. 2002). This is line with the results of this study, which showed that heavy metals decrease the size of vessels resulting in reduced stomatal conductance.
On the other hand, the results for wood rays width (Table 4) are completely different – in all clones higher values were determined at the contaminated site, while among clones grown at the control plot, the greatest wood rays width was recorded in clone 347 – 30.81 µm. It can be assumed that the investigated willow clones bind the largest amount of heavy metals at the contaminated soil in the cell wall and thus prevent further transport of heavy metals cations and their entry into the cytosol. The cell wall, especially with hyperaccumulator species, represents a physical and physiological barrier for further transport of metal ions due to the existence of a large number of carbohydrates rich in carboxyl groups such as pectin and lignin (Arsenov 2018). Therefore, heavy metals, as compounds of high specific gravity, lead to the narrowing of conducting elements – vessels, and as a result of increased heavy metals concentration, significantly wider wood rays appear, representsing an alternative conducting path for the rapid transport of water and mineral substances. Also, the greater wood rays width means a greater contact surface with the vessels, and thus the formation of a greater number of simple pits that have the role of transporting water and minerals against the forces of gravity. Metals may reduce the number of vessels by closing them gradualy, which negatively affects the process of transport through conductive tissues (Lamoreux and Chaney 1977). They can also significantly slow down the water uptake preventing the root cells to divide and elongate (Kahle 1993). Lower heavy metals content in the sapwood was explained by transport of toxic elements through the rays into the heartwood (Stewart 1966). There are specialized contact cells between wood rays and xylem vessels that serve for exchanging disolved substances between them (Sauter 1972). These cells are characterized by enlarged pits in contact with the xylem vessels and their respiratory and phosphatase activity raises in the vegetation season when differentation processes in the plant organisms are very intensive (Nabais et al. 1999). The active radial transport of elements is limited to the living sapwood cells and it stops existing at the sapwood-heartwood border where the ray cells die in the process of transformation of sapwood to heartwood (Stewart 1966).
Comparison of the wood rays height (Table 4) at both sites showed that higher levels were recorded only in Salix viminalis in the contaminated area. Among plants grown at the control plot, the highest value was observed in clone NS 73/6 (355 µm). The results confirmed the well-known fact that the width and height of wood rays are most often in an inversely proportional relationship (Vilotić 2000).
Table 5 Pearson's correlation coefficients between stem basal diameter and stem height and studied wood anatomical characteristics assessed in control and heavy-metal treatments
|
Characteristic |
Stem basal diameter |
Stem height |
Stem basal diameter |
Stem height |
|
Control |
Treatment |
|||
|
Fiber length |
0.895 ns |
0.781 ns |
0.995** |
0.997** |
|
Fiber diameter |
0.982* |
0.971* |
0.893 ns |
0.842 ns |
|
Fiber lumen diameter |
0.983* |
0.975* |
0.997** |
0.998** |
|
Double cell-wall thickness |
0.979* |
0.998** |
0.987* |
0.965* |
|
Vessel diameter |
0.999*** |
0.970* |
0.938 ns |
0.969* |
|
Wood rays width |
0.957* |
0.937 ns |
0.896 ns |
0.838 ns |
|
Wood rays height |
0.934 ns |
0.974* |
0.851 ns |
0.824 ns |
|
ns – non-significant; (*) p<0.05; (**) p<0.01; (***) p<0.001 |
||||
To identify characteristics that can be used as indicators of willow clones productivity under control and stress environments, the correlations between stem basal diameter and stem height, on one side, and studied wood anatomical characteristics, on the other, were assessed (Table 5). In control treatment, statistically significant correlations were observed for majority of characteristics. On the other side, in plants exposed to heavy metals, none of stem biometrical characteristics were correlated with fiber diameter and wood rays width and height. In addition, no significant correlation was found between SBD and VS.
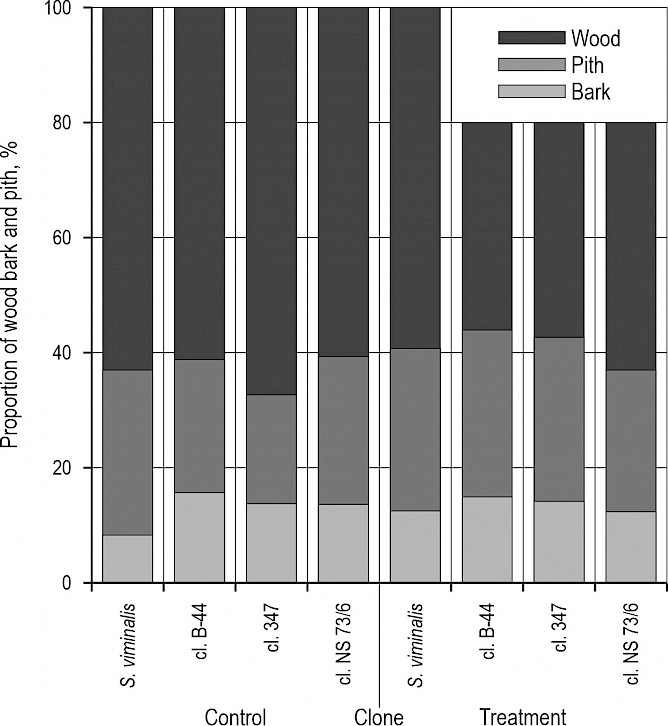
Fig. 1 Display of wood, bark and pith proportion by different willow clones at two sites
The proportion of different plant tissues (wood, bark and pith) differ between treatments and clones (Fig. 1). Namely, the proportion of wood and bark was higher in plants in the control treatment, while the situation was quite the opposite in the case of pith. At the clone level, S. alba cl. 347 had the highest proportion of wood (70.4%) and the lowest proportion of pith (17.1%) in the control treatment. Interestingly, clone NS 73/6 showed an opposite performance under two treatments; i.e. in the control treatment, this clone had the lowest wood (60.9%) and the highest pith proportion (27.1%), while in the heavy metal treatment it had the highest wood proportion (63.3%) and the lowest proportions of pith (26.5%) and bark (10.2%). Evidently, willows, as hyperaccumulators, under the influence of the increased heavy metals concentration, respond with a greater production of pith in the juvenile stage (Fig. 1). On the other hand, heavy metals have an inhibitory effect on the division and differentiation of cambial cells, which is why the secondary xylem proportion was significantly higher at the control plot in the first three clones, and slightly lower in the clone N 73/6 (Fig. 1). Mleczek et al. (2009) examined the accumulation of selected heavy metals by Salix viminalis cuttings in different soil conditions (non-polluted, moderate polluted and very polluted area) and an increase of bark content was recorded, but a decrease of the pith proportion independent on site conditions which is not in line with the results of our paper where at the contaminated site the proportion of pith increases, while the proportion of bark decreases. They also found the highest decrease of pith (17%) at most polluted area, while in this study the pith content increases according to heavy metals effects. There are some papers (Okada et al. 1990, Chun and Hui-Yi 1992) which established that the location of heavy metals is directly linked to the position of the sapwood-heartwood border. For example, some toxic elements such as Cd and Pb show growing tendency going from the cambium to the stem center (Nabais et al. 1999). Gardiyehewa de Silva et al. (2012) investigated the density of conductive elements by red maple stems under the stress influence and found that its reduction is related with increased number of parenchyma cells in the xylem.
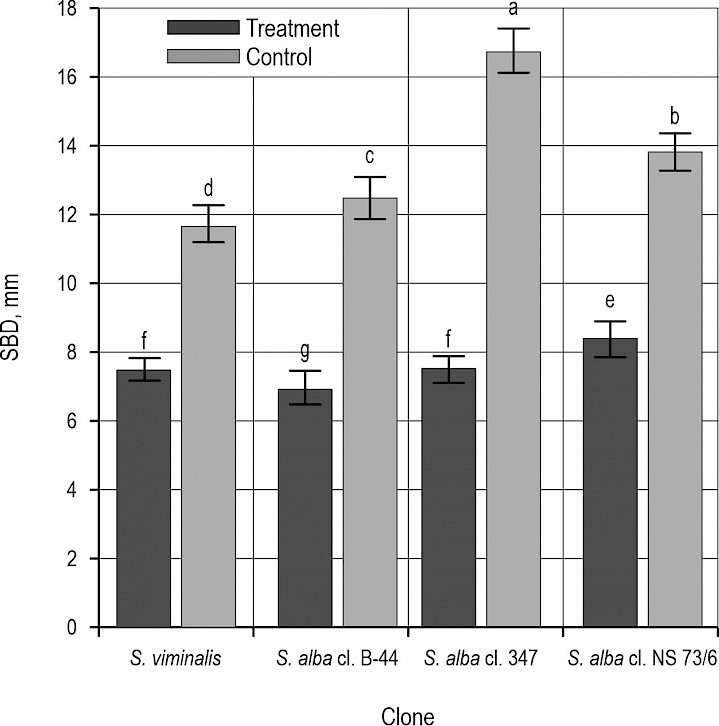
Fig. 2 Display of stem basal diameter by different willow clones at two sites
Considering biometrical characteristics (SDB and SH), significantly higher mean values were observed in the plants grown in the control treatment, whereby the S. alba cl. 347 was characterized by the highest mean values of both parameters (Fig. 2 and 3). Interestingly, plants of this clone also experienced the highest stress when subjected to heavy metals. Namely, although the plants of S. alba cl. B-44 showed the lowest mean values of both SDB and SH (Fig. 2 and 3), the strongest inhibitory effect of heavy metal pollution was recorded in S. alba cl. 347 (i.e. the plants stressed with heavy metals had smaller values of SDB and SH by 54.9% and 51.2%, respectively). The lowest reduction of SDB and SH were evidenced in S. viminalis and S. alba cl. NS 73/6, and amounted 37.8% and 26.4%, respectively. Similar findings were reported by Gardiyehewa de Silva et al. (2012), who found that combined effect of drought and heavy metals significantly reduced the growth of red maple seedlings. Similarly, Mulenga et al. (2022) established slower radial increment of the stems on the polluted compared to the control site. Some authors (Emamverdian et al. 2015, Kabata-Pendias 2010) believe that environmental stress caused by heavy metals pollution was responsible for less intensive division and differentation of the cambial cells, so this leads to the slower radial increment compared to the control site. Some papers (Zasoski et al. 1990, Mulenga et al. 2022) linked high heavy metals concentration and soil acidity from one side with growth patterns and adaptation mechanisms of the plants from another side at both control and contaminated site. Several papers (Zhang 2003, Krutul et al. 2014) found that wood quality is dependent on many factors such as tree species, age, position in the stand, radial and height growth, site conditions, etc. Kusiak et al. (2020) discovered that heavy-metals induced stress affects the reduction of wood cell dimensions which causes slower radial increment and forming of narrower annual rings. As a result, wood density increases according to higher uptake and accumulation of heavy metals.
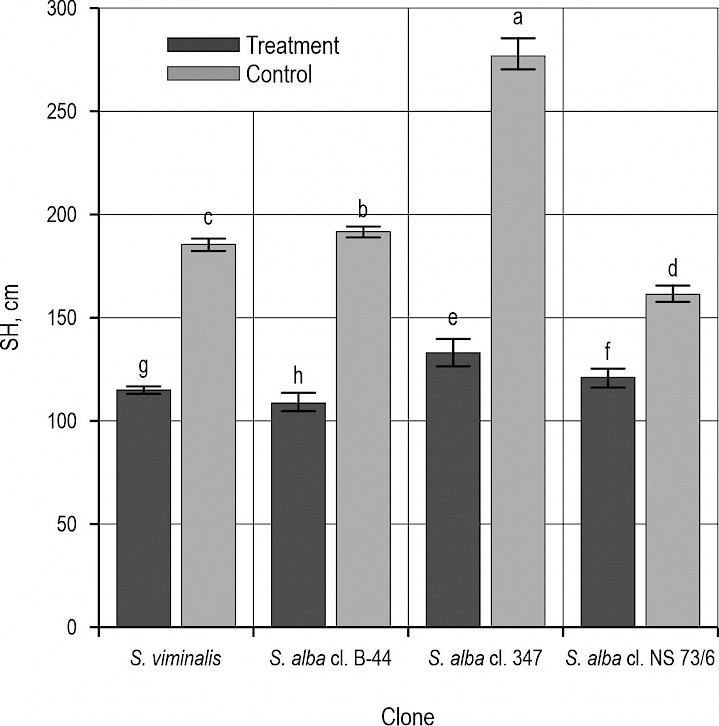
Fig. 3 Display of stem height by different willow clones at two sites
Tendel and Wolf (1988) examined the concentrations of sulphur in wood and found its direct relation to increased content of SO2 in the atmosphere. Pb concentration recorded in an annual growth ring is the readily ionic form fraction, but not the whole transportable content of Pb (Lepp and Dollard 1974). Ferretti et al. (1993) observed fluctuations of Ni concentrations and concluded that if growth is without significant deviations, its content in all annual rings is the same, so the availability of Ni does not change significantly with time. Hagemeyer (1995) tried to define mobile peaks of Cd at the sapwood-heartwood boundaries in stems of Quercus robur trees, but the locations of these peaks could not find out when exactly in the past this pollution in the tree occured.
4. Conclusions
This study investigated the impact of heavy metal induced stress on the morpho-anatomical characteristics of Salix clones wood. The results demonstrated that metal toxicities significantly affected dimensions of morphological and anatomical characteristics of investigated individuals. Considering morphological parameters, a significant reduction not only of radial and height increment, but also of bark and wood proportion (apart from clone NS 73/6) in all examined clones at the polluted site was detected. The analysis of anatomical characteristics showed that increased concentrations of heavy metals had a negative effect on vessel diameter and wood rays height (with the exception of Salix viminalis), but positively on wood rays width. For all wood fiber parameters higher values were obtained at the control site except for fiber lumen width where higher values were recorded at the polluted site in clones B-44 and NS 73/6. Between all the clones, it can be deduced that clone NS 73/6 had the highest values for 4 anatomical characteristics (fiber length, double cell-wall thickness, vessel diameter and wood rays height) both in control and contaminated conditions. Consequently it can be assumed that this will have a positive effect on the wood quality and utilization possibilities of this clone. At the control plot the greatest values for fiber lumen and fiber lumen diameter were found in Salix viminalis, while clone 347 had the widest wood rays. In control conditions, Pearson's correlation coefficients showed statistically significant correlations between stem basal diameter and stem height on one side and observed wood anatomical characteristics on the other side. However, in plants exposed to heavy metals stress, none of stem biometrical characteristics were correlated with fiber diameter and wood rays height and width. Clone 347 was characterized by the highest mean values of both stem basal diameter and stem height, but the strongest inhibitory effect of heavy metals pollution was recorded by this clone. As for the proportion of pith, bark and wood, clone 73/6 showed opposite performances under two treatments – i.e. in the control treatment this clone had the lowest wood and the highest pith proportion, while in the heavy metal treatment it had the highest wood proportion and the lowest proportion of pith and bark. It should also be noted that heavier texture composition of the contaminated soil and its weaker water retention properties may be one of the reasons for observed differences in wood anatomical characteristics. These results could make an undoubted contribution to the understanding of the behaviour of juvenile willow cuttings under the influence of increased heavy metals concentrations, as well as how it may affect their morpho-anatomical and ecophysiological characteristics.
5. References
Ahmad, S.H., Reshi, Z., Ahmad, J., Iqbal, M., 2005: Morpho-anatomical responses of Trigonella foenum graecum Linn. to induced cadmium and lead stress. J Plant Biol 48(1): 64–84. https://doi.org/10.1007/BF03030566
Alloway, B.J., 1995: Heavy metals in soils. Glasgow, Blackie Academic and Professional.
Antić, M., Jović, N., Avdalović, V., 1982: Pedologija. Naučna knjiga, Beograd, 380 p.
Arsenov, D., 2018: Physiological aspects of the potential of willows in assisted phytoremediation of cadmium using citric acid. University of Novi Sad, PhD thesis, 158 p.
Barceló, J., Cabot, C., Poschenrieder, C., 1986: Cadmium-induced decrease of water stress resistance in bush bean plants (Phaseolus vulgaris L. cv. Contender). II. Effects of Cd on endogenous abscisic acid levels. Journal of Plant Physiology 125(1–2): 27–34. https://doi.org/10.1016/S0176-1617(86)80240-1
Baum, S., Bolte, A., Weih, M., 2012: Short Rotation Coppice (SRC) Plantations Provide Additional Habitats for Vascular Plant Species in Agricultural Mosaic Landscapes. Bio-Energy Research 5: 573–583. https://doi.org/10.1007/s12155-012-9195-1
Carlquist, S., 1977: Wood anatomy of Onagraceae: Additional species and concepts. Annals of the Missouri Botanical Garden 64(3): 627–637. https://doi.org/10.2307/2395258
Chaney, R.L., Angle, J.S., Broadhurst, C.L., Peters, C.A., Tappero, R.V., Sparks, D.L., 2007: Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. Journal of Environmental Quality 36(5): 1429–1443. https://doi.org/10.2134/jeq2006.0514
Christersson, L., 2013: Papperspopplar och energipilar. Tranås: Budgetboken.
Chun, L., Hui-Yi, H., 1992: Tree-ring element analysis of Korean pine Pinus koraiensis Sieb. et Zucc. and Mongolian oak Quercus mongolica Fisch. ex Turcz. from Changbai Mountain, northeast China. Trees 6: 103–108. https://doi.org/10.1007/BF00226588
De Melo, J.C.F., Amorim, M.W., Soffiatti, P., 2018: Comparative wood anatomy of Ficus cestrifolia (Moraceae) in two distinct soil conditions. Rodriguesia 69(4): 2109–2118. https://doi.org/10.1590/2175-7860201869440
Emamverdian, A., Ding, Y., Mokhberdoran, F., Xie, Y., 2015: Heavy metal stress and some mechanisms of plant defense response. Sci World J 1: 756120. https://doi.org/10.1155/2015/756120
Ferretti, M., Udisti, R., Barbolani, E., 1993: Mineral nutrients and trace metals in tree rings of Pinus sp. Fresenius J Anal Chem 347: 467–470. https://doi.org/10.1007/BF00635481
Franklin, G.L., 1945: Preparation of thin sections of synthetic resins and wood-resin composites and a new macerating method for wood. Nature 155(3924): 51. https://doi.org/10.1038/155051a0
Gardiyehewa de Silva, N., Cholewa, E., Ryser, P., 2012: Effects of combined drought and heavy metal stresses on xylem structure and hydraulic conductivity in red maple (Acer rubrum L.). Journal of Experimental Botany 63(16): 5957–5966. https://doi.org/10.1093/jxb/ers241
Gupta, M.C., Iqbal, M., 2005: Ontogenetic histological changes in the wood of mango Mangifera indica L cv Deshi exposed to coal-smoke pollution. Environ Exp Botany 54(3): 248–255. https://doi.org/10.1016/j.envexpbot.2004.09.003
Hagemeyer, J., 1995: Radial distributions of Cd in stems of oak trees Quercus robur L. reanalyzed after 10 years. Trees 9: 200–203. https://doi.org/10.1007/BF00195273
Husen, A., Iqbal, M., 1999: Structural, functional and biochemical responses of Datura innoxia Mill. to coal-smoke pollution. Proc Acad Environ Biol 8: 61–72.
IAWA Committee, 1989: IAWA List of microscopic features for hardwood identification by an IAWA Committee. IAWA Bulletin 10: 219–332.
Iqbal, M., Mahmooduzzafar, A.I.M., Khan, P.R., 2010: Behavioral responses of leaves and vascular cambium of Prosopis cineraria (L.) Druceto different regimes of coal-smoke pollution. J Plant Interactions 5(2): 117–133. https://doi.org/10.1080/17429140903438084
Kabata-Pendias, A., 2010: Trace elements in soils and plants. Taylor & Francis.
Kahle, H., 1993: Response of roots of trees to heavy metals. Environmental and Experimental Botany 33(1): 99–119. https://doi.org/10.1016/0098-8472(93)90059-O
Kim, Y.R., Yoon, J.K., Kim, T.S., Yang, J.E., Owens, G., Kim, R.K., 2015: Bioavailability of heavy metals in soils: definitions and practical implementation—a critical review. Environmental Geochemistry and Health 37(6): 1041–1061. https://doi.org/10.1007/s10653-015-9695-y
Krutul, D., Zielenkiewicz, T., Zawadzki, J., Radomski, A., Antczak, A., Drozdzek, M., 2014: Influence of urban environment originated heavy metal pollution on the extractives and mineral substances content in bark and wood of oak (Quercus robur L.). Wood Research 59(1):177–190.
Kusiak, W., Majka, J., Ratajczak, I., Górska, M., Zborowska, M., 2020: Evaluation of environmental impact on selected properties of lime (Tilia Cordata Mill) wood. Forests 11(7): 746. https://doi.org/10.3390/F11070746
Lamoreaux, R.J., Chaney, W.R., 1977: Growth and water movement in Silver Maple seedlings affected by cadmium. Journal of Environmental Quality 6(2): 201–205. https://doi.org/10.2134/jeq1977.00472425000600020021
Lepp, N.W., Dollard, G.J., 1974: Studies on the behaviour of lead in wood. Binding of free and complexed 210 Pb to xylem tissue. Oecologia 16: 369–373. https://doi.org/10.1007/BF00344743
Mirck, J., Isebrands, J.G., Verwijst, T., Stig Ledin, S., 2005: Development of short-rotation willow coppice systems for environmental purposes in Sweden. Biomass and Bioenergy 28(2): 219–228. https://doi.org/ 10.1016/j.biombioe.2004.08.012
Mleczek, M., Magdziak, Z., Rissmann, I., Golinski, P., 2009: Effect of different soil conditions on selected heavy metal accumulation by Salix viminalis tissue. Journal of Environmental Science and Health 44(14): 1609–1616. https://doi.org/10.1080/10934520903263645
Mulenga, C., Clarke, C., Meincken, M., 2022: Effect of copper mining pollution-induced heavy metal toxicities on B. longifolia Benth wood cell characteristics. European Journal of Forest Research 142: 317–330. https://doi.org/10.1007/s10342-022-01524-x
Nabais, C., Freitas, H., Hagemeyer, J., 1999: Dendroanalysis: a tool for biomonitoring environmental pollution. The Science of the Total Environment 232(1–2): 33–37. https://doi.org/10.1016/s0048-9697(99)00107-2
Nikolić, N., 2009: Heavy metals influence on morpho-anatomical and physiological characteristics of poplar clones.University of Novi Sad, PhD thesis, 243 p.
Okada, N., Katayama, Y., Nobuchi, T., Ishimaru, Y., Aoki, A., 1990: Trace elements in the stems of trees IV. Radial distribution in mizunara Quercus mongolica var. grosseserrata. Mokuzai Gakkaishi 36(2): 93–97.
Özden, S., Ennos, R., 2018: The mechanics and morphology of branch and coppice stems in three temperate tree species. Trees 32: 933–949. https://doi.org/10.1007/s00468-018-1687-y
Perfus-Barbeoch, L., Leonhardt, N., Vavasseur, A., Forestier, C., 2002: Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. The Plant Journal 32(4): 539–548. https://doi.org/10.1046/j.1365-313x.2002.01442.x
Poschenrieder, C., Barceló, J., 2004: Water relations in heavy metal stressed plants. In: Prasad M, ed. Heavy metal stress in plants from biomolecules to ecosystems, 2nd edn. Berlin: Springer, 249–263 p.
Przedpelska-Wasowicz, E.M., Wierzbicka, M., 2011: Gating of aquaporins by heavy metals in Allium cepa L. epidermal cells. Protoplasma 248: 663–671. https://doi.org/10.1007/s00709-010-0222-9
Pulford, I.D., Riddell-Black, D., Stewart, C., 2002: Heavy metal uptake by willow clones from sewage sludge-treated soil: the potential for phytoremediation. International Journal of Phytoremediation 4(1): 59–72. https://doi.org/10.1080/15226510208500073
Rajput, K.S., Rao, K.S., Kim, Y.S., 2008: Cambial activity and wood anatomy in Prosopis spicigera (mimosaceae) affected by combinedair pollutants. IAWA J 29(2):209–219. https://doi.org/10.1163/22941932-90000180
Rajput, K.S., Rao, K.S., 2005: Cambial periodicity and formation of wood in Ailanthus excelsa growing under the influence of combined airpollutants. Phyton - Annales Rei Botanicae 45(1): 51–64.
Ross, S.M., 1994: Toxic metals in soil-plant systems. Chichester, John Wiley and Sons.
Safdari, V., Ahmed, M., Devall, M.S., Bayramzadeh, V., 2012: Effects of air pollution on morphological and anatomical characteristics of Pinus eldarica wood. Forest Journal of Biology 2(2): 5–12.
Sauter, J.J., 1972: Respiratory and phosphatase activities in contact cells of wood rays and their possible role in sugar secretion. Z Pflanzenphysiol 67(2): 135–145. https://doi.org/10.1016/S0044-328X(72)80127-2
Seregin, I.V., Kozhevnikova, A.D., 2008: Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel and strontium. Russian Journal of Plant Physiology 55: 1–22. https://doi.org/10.1134/S1021443708010019
Stewart, C.M., 1966: Excretion and heartwood formation in living trees. Science 153(3740): 1068–1074. https://doi.org/10.1126/science.153.3740.1068
Tendel, J., Wolf, K., 1988: Distribution of nutrients and trace elements in annual rings of pine trees Pinus silvestris as an indicator of environmental changes. Experientia 44: 975–980. https://doi.org/10.1007/BF01939892
Vilotić, D., 2000: Uporedna anatomija drveta. Šumarski fakultet, Univerzitet u Beogradu, 176 p.
Wali, B., Iqbal, M., 2007: Anatomical and functional responses of Calendula officinalis L. to SO2 stress as observed at different stages of plant development. Flora-Morphology, Distribution, Functional Ecology of Plants 202(4): 268–280. https://doi.org/10.1016/j.flora.2006.08.002
Yaltirik, F., 1971: Taxonomical Study on the Macro- and Micro- Morphological Characteristics of Indigenous Maples (Acer L.) in Turkey. Istanbul, Istanbul University Press, 232 p.
Zasoski, R.J.I., Porada, H.J., Ryan, P.J., Gessep, S.P., 1990: Observations of copper, zinc, iron and manganese status in western Washingtonforests. For Ecol Manage 37(1–3): 7–25. https://doi.org/10.1016/0378-1127(90)90043-B
Zimmermann, M.H., 1983: Xylem structure and the ascent of sap. Springer. https://doi.org/10.1007/978-3-662-22627-8
Zhang, S., 2003: Wood Quality Attributes and Their Impacts on Wood Utilization. XII World Forestry Congress, Quebec City, Canada. https://www.fao.org/3/xii/0674-b1.htm
© 2023 by the authors. Submitted for possible open access publication under the
terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Authors' addresses:
Assoc. prof. Dušan Jokanović, PhD
e-mail: dusan.jokanovic@sfb.bg.ac.rs
Department of Forestry and Nature Protection
University of Belgrade
Faculty of Forestry
Kneza Višeslava 1
11030 Belgrade
SERBIA
Assoc. prof. Vesna Nikolić Jokanović, PhD
e-mail: vesna.nikolic@sfb.bg.ac.rs
Department of ecological engineering for soil and water resources protection
University of Belgrade
Faculty of Forestry
Kneza Višeslava 1
11030 Belgrade
SERBIA
Jelena Urošević, MSc
e-mail: urosevicj75@gmail.com
Public Enterprise »EPS«
Department for biomass
Bogoljuba Uroševića Crnog 44
11 500 Obrenovac
SERBIA
Srđan Stojnić, PhD
e-mail: srdjan.stojnic@uns.ac.rs
Institute for Lowland Forestry and Environment
Antona Čehova 13d
21102 Novi Sad
SERBIA
Dragica Stanković, PhD
e-mail: dstankovic@imsi.bg.ac.rs
University of Belgrade
Institute for Multidisciplinary Research
Department of Plant-Soil and Nano Systems
Kneza Višeslava 1
11030 Belgrade
SERBIA
Assistant prof. Iva Ištok, PhD *
e-mail: iistok@sumfak.unizg.hr
Faculty of Forestry and Wood Technology
Institute of wood science
Svetošimunska cesta 23
10000 Zagreb
CROATIA
* Corresponding author
Received: February 21, 2023
Accepted: April 26, 2023
Original scientific paper

