Forest Residue Management Impact on Rodent (Rodentia: Murinae, Arvicolinae) Damage in Pedunculate Oak (Quercus robur L.) Forests in Croatia
doi: 10.5552/crojfe.2023.2028
volume: 44, issue:
pp: 15
- Author(s):
-
- Vucelja Marko
- Bjedov Linda
- Tomljanović Kristijan
- Kranjec Orlović Jelena
- Boljfetić Marko
- Matijević Mislav
- Margaletić Josip
- Article category:
- Original scientific paper
- Keywords:
- small rodents, forest residue management, rodent management, damage, lowland forests
Abstract
HTML
Small rodents (Rodentia, subfam. Murinae: real mice, Arvicoline voles) greatly affect natural regeneration, stability and dynamics of forest communities worldwide. Every 3–4 years rodent damage in Croatian state forests is the most severe in forest regeneration stands, especially in pedunculate oak (Quercus robur L.) and narrow-leaved ash (Fraxinus angustifolia Vahl.) forests, where rodents can seriously impede natural regeneration by damaging seeds, stems and roots of saplings. These negative interactions are an even bigger challenge nowdays as pedunculate oak and narrow-leaved ash have become more vunerable in the last decades and are known as the most sensitive species of lowland forests in Croatia due to microclimatic and macroclimatic changes and the unfavourable interaction of a whole series of anthropogenic, abiotic and biotic factors. In the last 40 years, in Croatian state forests, rodent management consisted of monitoring and mainly rodenticide use. Trying to implement IPM (Integrated Pest Management) postulates into practice over the years, different prevention methods against small rodents were tested, but not many came to use. The aim of this research was to look into different logging residue management approaches and their effect on the rodent damage in two pedunculate oak forest regeneration stands in central Posavina in Croatia. Rodent damage on stem and root of tree saplings was recorded by visual inspection on three plots (5x5 m) with scattered logging residue, and one plot (5x5 m) with no residue at one micro-depression site (95 m a.s.l.), and on one micro-elevation (99 m a.s.l.) site. Plots with scattered logging residue represented a type of forest residue management in which logging debris (branches) is cut to smaller lengths and distributed evenly at the forest regeneration stand. Plot with no logging debris represented a residue management method in which wood mass is completely removed from the regeneration stand after felling. We counted, determined and inspected tree saplings found at chosen plots for rodent damage (on stem and roots) and also determined the average weight and moisture content of logging residue (branches around 5–7 cm in diameter) found at the site. In spring 2017, 3380 tree saplings (2978; 81% pedunculate oak, 7; 0.2% narrow-leaved ash and 395; 11.7% other deciduous species) were inspected for rodent damage. At micro-depression site, on a plot with no logging residue, only 13.4% of the saplings were damaged, while the average share of damaged saplings on three plots with scattered residue was more than six times higher; 87.8%. The average mass of the logging residue weighed at site with scattered residue was 10.14 kg kg/m2 and moisture content was 19.2%. At micro-elevation site, 25.4% of the saplings were damaged on a plot with no logging residue, while the average share of damaged saplings on three plots with scattered residue was two times higher; 51.4%. The average mass of the logging residue weighed at SRP 1–3 was 5.1 kg/m2. We also determined moderately strong positive correlation (R=0.69133) between the mass of logging residue and rodent damage and strong negative correlation (R=–0.89598) between wood moisture content of the logging residue and rodent damage. In years ahead, with unpredictable climate effects and potentially very variable small rodent dynamics, removing the logging residue after the felling could represent a residue management that contributes to a more effective and ecologically based rodent management. It could also become a usable preventive method within IPM and help prevent sever rodent damage, even during the outbreaks in pedunculate oak regeneration stands.
Forest Residue Management Impact on Rodent (Rodentia: Murinae, Arvicolinae) Damage in Pedunculate Oak (Quercus robur L.) Forests in Croatia
Marko Vucelja, Linda Bjedov, Kristijan Tomljanović, Jelena Kranjec Orlović, Marko Boljfetić, Mislav Matijević, Josip Margaletić
Abstract
Small rodents (Rodentia, subfam. Murinae: real mice, Arvicoline voles) greatly affect natural regeneration, stability and dynamics of forest communities worldwide. Every 3–4 years rodent damage in Croatian state forests is the most severe in forest regeneration stands, especially in pedunculate oak (Quercus robur L.) and narrow-leaved ash (Fraxinus angustifolia Vahl.) forests, where rodents can seriously impede natural regeneration by damaging seeds, stems and roots of saplings. These negative interactions are an even bigger challenge nowdays as pedunculate oak and narrow-leaved ash have become more vunerable in the last decades and are known as the most sensitive species of lowland forests in Croatia due to microclimatic and macroclimatic changes and the unfavourable interaction of a whole series of anthropogenic, abiotic and biotic factors. In the last 40 years, in Croatian state forests, rodent management consisted of monitoring and mainly rodenticide use. Trying to implement IPM (Integrated Pest Management) postulates into practice over the years, different prevention methods against small rodents were tested, but not many came to use. The aim of this research was to look into different logging residue management approaches and their effect on the rodent damage in two pedunculate oak forest regeneration stands in central Posavina in Croatia. Rodent damage on stem and root of tree saplings was recorded by visual inspection on three plots (5x5 m) with scattered logging residue, and one plot (5x5 m) with no residue at one micro-depression site (95 m a.s.l.), and on one micro-elevation (99 m a.s.l.) site. Plots with scattered logging residue represented a type of forest residue management in which logging debris (branches) is cut to smaller lengths and distributed evenly at the forest regeneration stand. Plot with no logging debris represented a residue management method in which wood mass is completely removed from the regeneration stand after felling. We counted, determined and inspected tree saplings found at chosen plots for rodent damage (on stem and roots) and also determined the average weight and moisture content of logging residue (branches around 5–7 cm in diameter) found at the site. In spring 2017, 3380 tree saplings (2978; 81% pedunculate oak, 7; 0.2% narrow-leaved ash and 395; 11.7% other deciduous species) were inspected for rodent damage. At micro-depression site, on a plot with no logging residue, only 13.4% of the saplings were damaged, while the average share of damaged saplings on three plots with scattered residue was more than six times higher; 87.8%. The average mass of the logging residue weighed at site with scattered residue was 10.14 kg kg/m2 and moisture content was 19.2%. At micro-elevation site, 25.4% of the saplings were damaged on a plot with no logging residue, while the average share of damaged saplings on three plots with scattered residue was two times higher; 51.4%. The average mass of the logging residue weighed at SRP 1–3 was 5.1 kg/m2. We also determined moderately strong positive correlation (R=0.69133) between the mass of logging residue and rodent damage and strong negative correlation (R=–0.89598) between wood moisture content of the logging residue and rodent damage. In years ahead, with unpredictable climate effects and potentially very variable small rodent dynamics, removing the logging residue after the felling could represent a residue management that contributes to a more effective and ecologically based rodent management. It could also become a usable preventive method within IPM and help prevent sever rodent damage, even during the outbreaks in pedunculate oak regeneration stands.
Keywords: small rodents, forest residue management, rodent management, damage, lowland forests
1. Introduction
Small rodents (order Rodentia Bowdich 1821, subfam. Murinae Illiger 1811: real mice and Arvicoline Gray 1821: voles) greatly affect natural regeneration, stability and dynamics of forest communities and have triggered numerous research regarding their ecological, economical and health impacts (Jacob and Tkadlec 2010, Singleton et al. 2010, Holland et al. 2015, Ostfeld et al. 1996, Meerburg et al. 2009a). Distributed worldwide, they are intelligent, partially subterranean, predominantly nocturnal, polyphagous mammals with extremely high reproduction potential and variable population dynamics (Henttonen 2000, Gliwicz 1980, Lambert 1985). Despite many of their »positive« environmental impacts (e.g. links/interactions in food chains/webs; seed dispersal, ecological succession, etc.) (Erlinge 1983, Pelz 2003, Bryja et al. 2002, Bartmann et al. 1992, Jacob and Tkadlec 2010), forest management mainly focuses on their »negative« aspects.
During the peaks or their outbreaks (every 3–4 years throughout Europe) (Hansson and Henttonen 1985, Delattre et al. 1992, Tkadlec and Stenseth 2001, Vucelja 2013), rodents can seriously impede natural forest regeneration by causing damage to seeds, stem and roots of saplings (Margaletić 1997, Margaletić et. al. 2007, Križančić 2012, Vucelja at al. 2014). Evidence of such periodical damage made by rodents (mainly on oak, ash, alder, hornbeam, willow and poplar) with different shares of damaged saplings (sometimes as high as 98%), has been reported in many European countries (Gill 1992, Rooney and Hayden 2002, Bäumler 1990, Hansson and Zejda 1977, Niemeyer and Haase 2003). Monitoring of small rodents in Croatian state forests has been carried out systematically for over 40 years by Croatian Forest Research Institute (CFRI) and »Hrvatske šume«, a limited liability company (HŠ LLC). Results of such monitoring show growing trends of rodent damage in lowland forests, indicating the increase in rodent numbers (PPSF 1980–2020). In the last twenty years (2001–2020), annual size of forest area on which rodent damage occurred has been extended to approx. 4000 hectares (ha) on average. While trying to prevent rodent damage, especially in young forest stands, it is important to be aware that rodent density fluctuations, and therefore damage, is the result of the interaction of numerous factors, including their community structure, food supply, number of predators, climatic and habitat conditions, forest management, etc. (Henttonen 2000, Gliwicz 1980, Huitu et al. 2013, Solonen 2004, Solonen 2001, Marsh and Harris 2000).
It is well known that weed control or the reduction of any undesirable vegetation and also the removal of logging residues makes the environment less suitable for the rodents (Hansson 1982, King 1985, Thompson and Pitt 2003, Sutton 1993, Davies and Pepper 1993, Jacob and Halle 2001, Huitu et al. 2003, Hytönen and Jylhä 2005, Nilsson and Örlander 1999, Birkedal et al. 2009, Vucelja 2013). From the beginning of the 1980s, control measures against small rodents in Croatian state forests have heavily relied on rodenticide use (cumulative; A.I. bromadiolone, difenacoum; acute; zinc phoshide; 8500 kg/yr and 3.0 kg/ha on average) (PPSF 1980–2020). There are also records of snap-traps, deratization glue and CO2 being used periodically, but on a much smaller scale. While different types of rodent repellents (e.g. audio-tactile, taste and smell repellents) have also been tested in the last ten years (Margaletić 2002, Vucelja 2013) – and some with rather promising efficiency – rodenticides are still a predominant control measure against rodents in Croatian forests, despite the periodical derogations that need to be approved (for rodenticide usage) by Forest Stewardship Council (FSC) for HŠ LLC as a certificate holder. No doubt that there is a need for shifting the focus from rodenticides usage to utilizing rodent control methods that would meet the ecological standards of FSC certificate. While introducing the new »Ecologically Based Rodent Management« approach, Singleton (1999) pointed out that understanding of rodent biology and social aspects of our common rodent species should provide us with guidelines we need to become more successful in preventing the damage caused by rodents. The aim of this research was to determine whether different residue management approaches affect the small rodent damage on saplings in pedunculate oak (Quercus robur L.) forest regeneration stands in Central Posavina in Croatia.
2. Materials and Methods
The aim of this study was to test the effects of different logging residue management approaches in two pedunculate oak (Quercus robur L.) forest regeneration stands in accordance with the rodent damage to tree saplings. Research was conducted during spring 2017 in floodplain forests in Croatian continental biogeographic region in central Posavina (Lipovljani, Opeke), situtated within the Lonjsko Polje Nature Park (Fig. 1).
Fig 1 Research site, Lipovljani (Opeke) in Central Posavina, Croatia
Two young-growth pedunculate oak forests chosen for this research were previously known for the severity of damage periodically caused by small rodents. These two regeneration stands are state owned forests under the management of »Hrvatske šume«, a limited liability public company for forest and woodland management in the Republic of Croatia. The Sava River, with its tributaries, is a predominant development factor in this area through underground water and flood waters. Floods occur 2–6 times a year with a total duration of 200–290 days on average and their height is usually around 99 m a.s.l., and occasionally 101 and 102 m a.s.l. The geological base is a Quaternary formation represented by alluvial deposits (a1) and marsh flagstone (Q1). The main types of soil are:
- alluvial soil (stratigraphic formulas of the soil profile: (A)–I–II, with 2 subtypes: (1a) carbonate soil up to 150 cm, (1b) carbonate soil up to 40 cm)
- swamp gley soil (eugleium) ((A) – I – II with subtypes (2a) hypogleic, (2b) epigleic, (2c) amphigleic)
- pseudogley (A- Eg – Bg – C).
According to Köpren and Thornthwaite's classification, this study site has the characteristics of a warm temperate rainy climate with the temperature of the coldest month not lower than –3oC, while cool summers record monthly temperatures of the warmest month below 22oC. The amount of precipitation is evenly distributed throughout the year and the driest period falls in the cold part of the year. The maximum amount of precipitation that appears at the beginning of the warm part of the year reaches the maximum amount in late autumn. According to the established Lang rain factor, this area is classified as a humid climate (KFg=88.55). Frost and snow appear in the cold season, but are always interrupted by warmer periods, so long-term snow cover is not a regular occurrence. The two chosen forest stands belonged to the same type of forest community (Genisto elatae – Quercetum roboris Ht. 1938) and are at the same developmental stage (young growth), but at different altitudes (94–96 m a.s.l.: micro depression, 99 m a.s.l.: micro elevation). On both sites, seed cuttings were done in the period from 2010 to 2012 and the final cutting was done in 2015. Between the two cuttings, different protection measures were taken against pests and diseases: fencing, rodenticide application (A.I. zink phosphide, 3 kg/ha on average), treating oak powdery mildew Erysiphe (Microsphaera) alphitoides (Griffon & Maubl.) U. Braun & S. Takam, weeding and cleaning). Pedunculate oak is a dominant tree species (70–80%) at both sites. Other tree species are narrow-leaved ash, common (black) alder (Alnus glutinosa (L.) Gaertn), field elm (Ulmus minor Mill), poplars (Populus spp.) and willows (Salix spp.) at SC 119b and common hornbeam (Carpinus betulus L.), small-leaved linden (Tilia cordata Mill.), field maple (Acer campestre L.) at SC 175a.
Field work included finding four research plots on every site. Area size of each plot was 25 m2 (5x5 m). At three plots on every site, forest residue management was done by equally scattering logging residues on forest ground surface (SRP: scattered residue plots). At one plot on every site, no logging residues were left after the felling, so the terrain was basically clear from the branches (NRP: no residue plot). Plots with no logging residue represented the favourable residue management approach in terms of rodent management. On every plot, all the tree saplings were counted, determined and inspected for rodent damage on roots and stem. Regarding tree species, saplings were categorized into three groups; the first one belonging to pedunculate oak (PO), the second to narrow-leaved ash (NLA) and the last to other hardwood or softwood broad-leaved species (ODSP). Regarding the damage, saplings were categorized into four groups; the first one being undamaged saplings, then those with damaged root, damaged stem or damaged root and stem.
The quantity of wood material, left after the shelterwood cuttings on the plots with scattered residue, was measured by weighing. First, branches were collected into piles, put on the tarpaulin and weighed with spring scale.
The moisture content of the same wood material was also measured. The procedure for determining the moisture content was a modified gravimetric method, where the amount of moisture is the difference between the mass of the fresh sample and the mass of the dry sample. The norm for the gravimetric method prescribes a minimum sample quantity of 300 g (HRN EN 14774-1:2010). Several representative samples were taken from each individual pile. The branches were chopped with a hand tool (axe), placed in a heat-resistant container with a bottom surface of 1 cm2 for each gram of the sample, and were weighed with a laboratory scale with a reading accuracy of 0.1 g in the fresh state directly in the forest stand. Before weighing the samples, it was necessary to weigh the empty containers to an accuracy of 0.1 g. The method of determining the moisture content requires a dryer with the ability to regulate the temperature at 105±2 °C and air exchange 3–5 times per hour. At the Faculty of Forestry and Wood Technology, University of Zagreb, at the Laboratory of Forest Biomass, the samples were dried in an dryer at 105±2 °C until constant mass was reached, which occurred after 24 hours (mass change not exceeding 0.2% of the total mass loss during the next drying period of 60 minutes). After drying, it was necessary to weigh the heated container with the sample to the nearest 0.1 g, within 10–15 seconds after it was removed from the dryer in order to avoid the absorption of atmospheric moisture due to the hygroscopicity of the analysed material. Moisture content (Mar) is calculated on a wet basis in biofuel expressed as mass fraction according to the formula (Zečić and Vusić 2016) shown later in the text. Collected data was analysed by Microsoft Office Excel 365.
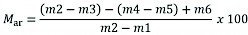
Where:
m1 mass of empty container, g
m2 mass of container and sample before drying, g
m3 mass of container and sample after drying, g
m4 mass of container and sample before drying at room temperature, g
m5 mass of container and sample after drying (weighed while hot), g
m6 mass of moisture in the container in which the sample was delivered, g.
3. Results
In spring 2017, at two pedunculate oak forest regeneration stands in Central Posavina, damage caused by small rodents was determined by visual inspection of 3380 tree saplings; 1805 (53.4%) at micro-depression (95 m a.s.l.) site, and 1575 (46.6%) at micro-elevation (99 m a.s.l) site. Pedunculate oak was the dominant species at both sites (93.33% at micro-depression, and 82.2% at micro-elevation site).
Table 1 shows the data on inspected tree samplings regarding determined tree species and rodent damage on a plot with no forest residue and on the plots with scattered forest residue recorded at micro-depression site (95 m a.s.l.). According to data collected at micro-depression site, there was great difference in total number of saplings between the plot free from residue (1069) and the plots with scattered residue. If we take into account the average number of saplings counted on plots with scattered residue (N=233), it is more than four times lower than the number of saplings found on a plot that is clear from the felling debris. When considering the share of damaged saplings at micro-depression site, on a plot with no residue (13.4%) it is more than 6 times lower compared to an average share of damaged sapling on the plot with the logging residue (87.8%), while the share of undamaged saplings (86.6%) on that same plot is 7 times higher than the average share of saplings found on plots with scattered residue (12.2%).
Table 1 Rodent damage on tree saplings in pedunculate oak forest regeneration stand (95 m a.s.l.; micro-depression) in central Posavina region (Lipovljani, Opeke)
|
Research plot (NRP: no logging residue plot; SRP 1–3: scattered residue plots) |
Rodent damage on saplings |
Pedunculate oak PO |
Narrow-leaved ash NLA |
Other deciduous species ODSP |
∑ |
% |
|
NRP |
Undamaged |
899 |
0 |
27 |
926 |
86.6 |
|
Damaged stem |
69 |
0 |
24 |
93 |
8.7 |
|
|
Damaged root |
48 |
0 |
2 |
50 |
4.7 |
|
|
Damaged root and stem |
0 |
0 |
0 |
0 |
0,0 |
|
|
∑ |
1016 |
0 |
53 |
1069 |
100 |
|
|
SRP 1 |
Undamaged |
0 |
0 |
0 |
0 |
0.0 |
|
Damaged stem |
106 |
0 |
19 |
125 |
61.6 |
|
|
Damaged root |
39 |
0 |
3 |
42 |
20.7 |
|
|
Damaged root and stem |
33 |
0 |
3 |
36 |
17.7 |
|
|
∑ |
178 |
0 |
25 |
203 |
100 |
|
|
SRP 2 |
Undamaged |
127 |
0 |
3 |
130 |
33.1 |
|
Damaged stem |
129 |
0 |
12 |
141 |
35.9 |
|
|
Damaged root |
91 |
0 |
1 |
92 |
23.4 |
|
|
Damaged root and stem |
30 |
0 |
0 |
30 |
7.6 |
|
|
∑ |
377 |
0 |
16 |
393 |
100 |
|
|
SRP 3 |
Undamaged |
3 |
0 |
2 |
5 |
3.6 |
|
Damaged stem |
58 |
5 |
19 |
82 |
58.6 |
|
|
Damaged root |
27 |
0 |
0 |
27 |
19.3 |
|
|
Damaged root and stem |
25 |
0 |
1 |
26 |
18.6 |
|
|
∑ |
113 |
5 |
22 |
140 |
100 |
|
|
∑∑ |
1684 |
5 |
116 |
1805 |
53.4 |
|
The above differences regarding the number of saplings and the share of damaged saplings are not so distinct at micro-elevation site (99 m a.s.l.). Table 2 shows the data on inspected tree samplings regarding the determined tree species and rodent damage on a plot with no forest residue and on the plots with scattered forest residue recorded at micro-elevation site (99 m a.s.l.). The difference between the number of saplings counted on a plot with no logging residue (N=471) compared to the number of saplings found on a plot that with scattered debris (368) at micro-elevation site was not as high as on a micro-depression site.
Among the damaged saplings found at micro-depression site, on a plot with no logging residue, 65% had damaged stem, and 35% damaged roots. Among the damaged saplings found on a plot with scattered residue, 58.6% had damaged stem and 41.4% had damaged roots on average.
At a micro-elevation site, those ratios were quite different. Among the damaged samplings found on a plot clear from residue, 95.9% had damaged stem, and only 4.1% had damaged roots. On plots with scattered logging residue, 90.2% of damaged samplings had damaged stem, and 9.8% damaged roots.
Shares (%) of the inspected saplings un/damaged by small rodents on all research plots at micro-depression (95 m a.s.l.) and micro-elevation (99 m a.s.l.) sites, together with average mass of logging residue weighed on each research plot (kg/m2), are shown in Fig. 2.
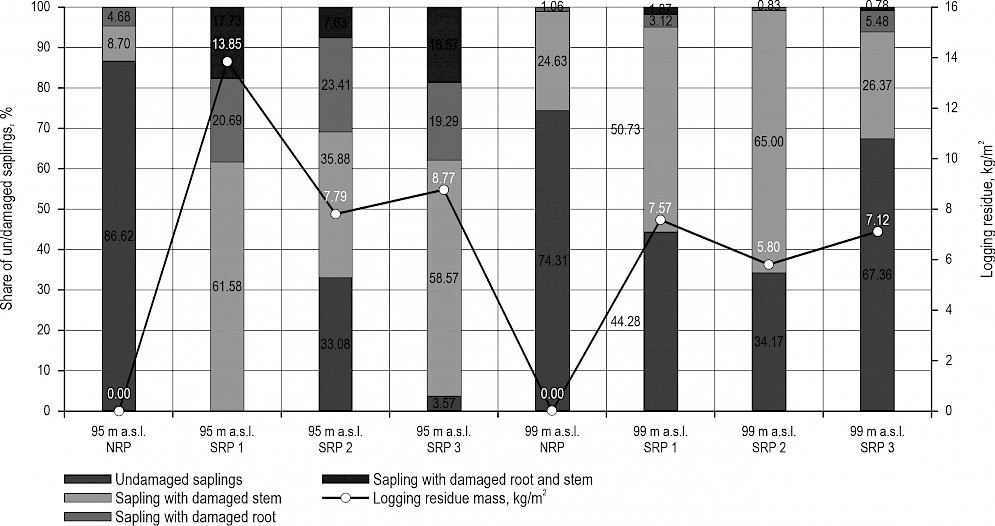
Fig. 2 Rodent damage on tree saplings on plot with no logging residue (NRP) and on scattered residue plots (SRP 1–3) at micro-depression (95 m a.s.l.) and micro-elevation (99 m a.s.l.) sites in relation to mass (kg/m2) of logging residue found on research plots
Table 2 Rodent damage on tree saplings in pedunculate oak forest regeneration stand (99 m.a.s.l.; micro-elevation) in central Posavina region (Lipovljani, Opeke)
|
Research plot (NRP: no logging residue plot; SRP 1-3: scattered residue plots) |
Rodent damage on saplings |
Pedunculate oak PO |
Narrow-leaved ash NLA |
Other deciduous species ODSP |
∑ |
% |
|
NRP |
Undamaged |
285 |
0 |
65 |
350 |
74.31 |
|
Damaged stem |
65 |
1 |
50 |
116 |
24.63 |
|
|
Damaged root |
5 |
0 |
0 |
5 |
1.06 |
|
|
Damaged root and stem |
0 |
0 |
0 |
0 |
0 |
|
|
∑ |
355 |
1 |
115 |
471 |
100 |
|
|
SRP 1 |
Undamaged |
183 |
0 |
30 |
213 |
44.28 |
|
Damaged stem |
204 |
1 |
39 |
244 |
50.73 |
|
|
Damaged root |
15 |
0 |
0 |
15 |
3.12 |
|
|
Damaged root and stem |
9 |
0 |
0 |
9 |
1.87 |
|
|
∑ |
411 |
1 |
69 |
481 |
100 |
|
|
SRP 2 |
Undamaged |
29 |
0 |
53 |
82 |
34.17 |
|
Damaged stem |
131 |
0 |
25 |
156 |
65 |
|
|
Damaged root |
2 |
0 |
0 |
2 |
0.83 |
|
|
Damaged root and stem |
0 |
0 |
0 |
0 |
0 |
|
|
∑ |
162 |
0 |
78 |
240 |
100 |
|
|
SRP 3 |
Undamaged |
242 |
0 |
16 |
258 |
67.36 |
|
Damaged stem |
100 |
0 |
1 |
101 |
26.37 |
|
|
Damaged root |
21 |
0 |
0 |
21 |
5.48 |
|
|
Damaged root and stem |
3 |
0 |
0 |
3 |
0.78 |
|
|
∑ |
366 |
0 |
17 |
383 |
100 |
|
|
∑∑ |
1294 |
2 |
279 |
1575 |
46.6 |
|
Shares (%) of damaged pedunculate oak (PO) and other deciduous species (ODSP) on all research plots at micro-depression (95 m a.s.l.) and micro-elevation (99 m a.s.l.) sites, together with average mass of logging residue weighed on each research plot (kg/m2), are presented in Fig. 3. and Fig. 4.
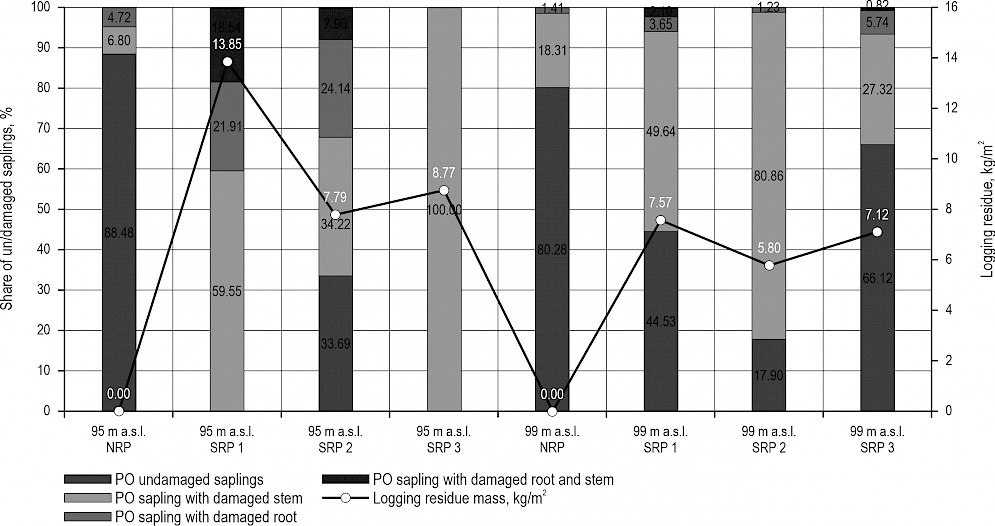
Fig. 3 Rodent damage on pedunculate oak (PO) saplings on plot with no logging residue (NRP) and on scattered residue plots (SRP 1–3) at micro-depression (95 m a.s.l.) and micro-elevation (99 m a.s.l.) sites in relation to mass (kg/m2) of logging residue found on research plots
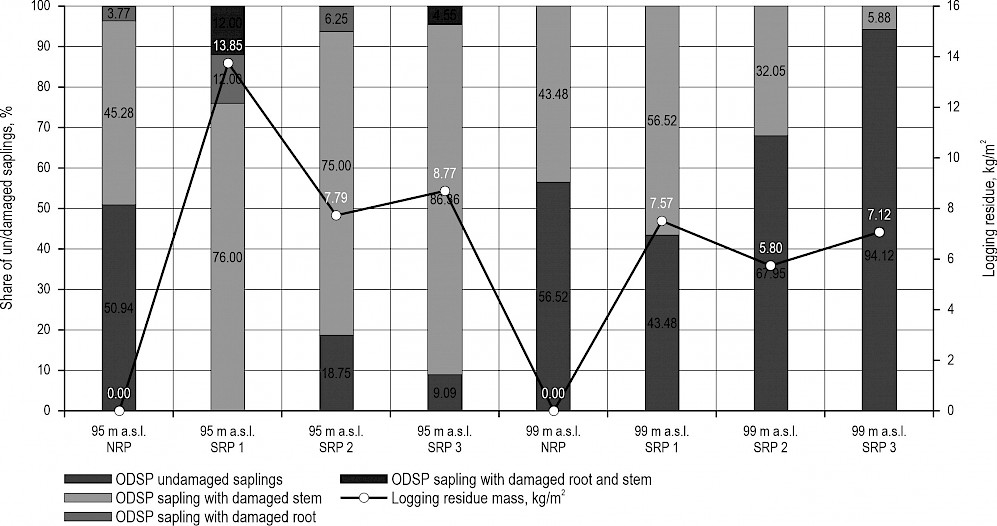
Fig. 4 Rodent damage on other deciduous saplings (ODSP) on plot with no logging residue (NRP) and on scattered residue plots (SRP 1–3) at micro-depression (95 m a.s.l.) and micro-elevation (99 m a.s.l.) sites in relation to mass (kg/m2) of logging residue found on research plots
Mass of the logging residue (branches thicker than 5–7 cm on average) collected on research plots with scattered residue (SRP 1–3), at both micro-depression (95 m a.s.l.) and micro-elevation site (99 m a.s.l.), and wood moisture content (%) of the same residue are shown in Table 3. Correlation between the average mass of logging residue (kg/m2), residue wood moisture content (%) and share of the saplings damaged by small rodents on different experimental plots, is shown in Table 4.
Table 3 Logging residue mass (kg) and moisture content (%) found and weighed on research plots with scattered logging residue (SRP 1–3) in two pedunculate oak forest regeneration stands (95 m a.s.l.; micro-depression, 99 m a.s.l.; micro-elevation) in central Posavina region (Lipovljani, Opeke)
|
Research plots with scattered logging residue (SRP 1–3) at micro-depression and micro-elevation site |
Mass of logging residue found and weighed on a plot kg/25m2 |
Aver. logging residue mass per plot kg/m2 |
Residue wood moisture content (%) determined in laboratory |
|
95 m a.s.l. SRP 1 |
346.2 |
13.85 |
19.56 |
|
95 m a.s.l. SRP 2 |
194.8 |
7.79 |
26.67 |
|
95 m a.s.l. SRP 3 |
219.2 |
8.77 |
11.29 |
|
99 m a.s.l. SRP 1 |
188.9 |
7.57 |
29.37 |
|
99 m a.s.l. SRP 2 |
145.13 |
5.8 |
33.47 |
|
99 m a.s.l. SRP 3 |
177.7 |
7.12 |
37.09 |
Table 4. Correlation analysis (Pearson correlation coefficient; R)
|
|
Aver. logging residue mass per experimental plot, kg/m2 |
Wood moisture content, % |
Damaged samplings, % |
|
Aver. logging residue mass per experimental plot, kg/m2 |
1 |
– |
– |
|
Wood moisture content, % |
–0.584207662 |
1 |
– |
|
Damaged samplings, % |
0.691330831 |
–0.895989874 |
1 |
4. Discussion and Conclusions
Small rodents (Murinae:mice; Arvicolinae:voles) are not by any means a »new challenge« in Croatian forestry. Almost 150 years ago, in scientific-technical and professional journal of Croatia Forestry Society »Šumarski list« (No. 6, Year 5, 1881), the article named »Defects and damage from field mice« dealt with the topic of a rodent outbreak in Europe. Furthermore, monitoring of small rodents in the state forests of Croatia has been carried out systematically since the early 1980s. Trying to fit better into Integrated Pest Management (IPM) practices, in 2017 rodent monitoring was also added up to its methodology and now it includes trapping rodents as well as determining the share of damaged seeds and tree saplings, mostly in regeneration forest stands in Croatian continental bioregion (Vucelja et al. 2018). While IPM approach, besides continuous monitoring, also emphasizes the need for conducting primarily preventive, and then, only if necessary, the repressive measures of protection (EPA 2013), this research focused at forest residue management as one of preventive methods of forest protection. The concept of residue management was known and used in different situation to different extent in Croatian forestry over time (Ugrenović 1957). Vajda (1974) points out that maintaining forest residue management can help forest become more resilient to different biotic and abiotic threats. We challenged this statement by trying to investigate the effect of specific residue management approach on the damage made by small rodents in two pedunculate oak regeneration forest stands located in central Posavina region (Fig. 1.).
In this study, rodent damage on stem and root of tree saplings was recorded by visual inspection on three plots with scattered logging residue (SRP 1–3), and one plot with no residue (NRP) at one micro-depression (95 m a.s.l.) and one micro-elevation (99 m a.s.l.) site. Being a byproduct from harvesting (e.g. thinnig, shelterwood cutting, final cutting etc.) forest residue can be managed and used in different ways (Belyakov 2019, Väätäinen 2021). Despite their low density and heating values with high transportation cost, they are also major source of biomass, therefore management of forest residue can contribute minimizing the environmental impact and increase their sustainability (Pergola et al. 2022). If not removed from the forest stand, forest residue can also be useful for ground protection and reducing soil disturbance when planning forest operations (e.g. driving on logging residue or logging mats can prevented exposure of mineral soil) (Ring et al. 2022). Forest residue left scattered on ground surface after the logging, also have an ecological effect regarding small rodent populations that are usually high at regeneration forest stands. Providing them with shelter against predators (especially predatory birds) (Solonen 2004, Walker 2008), forest residue may contribute to a higher rodent population (Vucelja 2013) and more severe rodent damage on different tree saplings of forest young growth. Within this research, plots with scattered logging residue (SRP) represented a forest residue management type in which logging debris (branches) is cut to smaller lengths and distributed evenly at the forest regeneration stand (Glavaš 2017). This forest residue method is sometimes used during shelterwood cuttings by »Hrvatske šume«, a limited liability public company for forest and woodland management in the Republic of Croatia. The plot with no logging debris (NRP) represented a residue management method in which wood mass is completely removed from the regeneration stand after felling. In terms of rodent management, the latter should be the most desirable forest residue management method. Within this research, 3380 saplings were inspected, with pedunculate oak (Quercus robur L.) (PO) being the most dominant species (2978; 88.1%). Only seven saplings of narrow leaved-ash (Fraxinus angustifolia Vahl.) (NLA) were found all together, and the remaining 11.7% of the saplings were recorded as other deciduous species (ODSP).
Taking into account all the research plots with and without logging residue, overall rodent damage on stem and roots of inspected tree saplings was a bit higher than 40% at both micro-depression (41.2%) and micro-elevation (42.7%) sites. These results correspond to rodent damage recorded at 10 different sites in forest-office Lipovljani, in the same year (Vucelja et al. 2018). According to the yearly report of Plant protection survey in forestry made by Croatian Forest Research Institute, the year 2017 was the year of rodent outbreak when rodent damage was counted at 5100 ha in Croatian state owned forests (PPSF 1980–2020). Vucelja et al. (2018) reported that the relative rodent abundance was determined by rodent sampling. Sampling was carried out 20 times at micro-depression site used in this research, and 18 times at micro-elevation site from April 2016 to June 2017, and the average abundance was 46% at micro-depression site and 50% at micro-elevation site. Such high abundances, indicating mass occurrence or the outbreak (Androić at al. 1981, Delany 1974, Crnković 1982), provide the explanation for high shares of damaged saplings recorded during this research. This also speaks in favour of ecological conditions that should be more suitable for small rodents at micro-elevations than at micro-depressions due to shorter flooding periods (Pietsch 1978, Henttonen 2000, Gliwicz 1980, Huitu et al. 2013). Rodents use the remains of tree branches and other wood material, as well as dense ground cover, shrubs, bushes and weedy vegetation for noiseless movement and as a natural shelter from a direct threat from predators (Kolb and Weisshaar 2005, Heroldova et al. 2007, Hytönen and Jylha 2005, Suchomel 2008, Davies and Pepper 1993, Ward 1993, Mujezinović 2010, King 1985, Hansson 1982). Considering the ratio of damaged and undamaged saplings at micro-depression site (Fig. 2.) – where the share of damaged saplings on the plot with no residue was 13.4%, in contrast to the average share recorded on plots with scattered residue amounting to 87.8% – it can be concluded that scattered logging residue on a forest floor increases the chances for rodent damage, while providing rodents with shelter. These results also match previous reports by Vucelja (2013), where number of active rodent entrance holes at the micro-depression site with scattered logging residue was 2.5 times higher than at a site clear from residue. On that same site, Stapić (2017) reported that rodent damage on saplings was about eight times higher on the plots with weedy vegetation than on plots free from weeds at lower elevation sites and almost three times higher at higher elevation sites. As an additional support to the above findings, we also determined the strength of the linear relationship between the average mass of logging residue, wood moisture content and rodent damage and as a result a moderately strong positive correlation (R=0.69133) was obtained between the mass of logging residue and rodent damage, and strong negative correlation (R=–0.89598) between wood moisture content (%) and rodent damage (Table 3 and 4). Having in mind that the number of tree saplings (N=1016) recorded on plots with no logging residue (NRP) at micro-depression site (SC 119b) was more than 4 times higher than the average number of saplings (N=223) (Table 1) inspected on plots with scattered residue, removing logging debris from the regeneration stands seems to contribute to a better survival of pedunculate oak young-growth. In terms of forest management, forest residue management – being a useful »rodent management tool« – becomes also important for financial reasons, as it lowers the price of artificial restoration at pedunculate oak forest stands. According to Anić (2007), for the artificial restoration of pedunculate oak, it is necessary to sow 700–1000 kg of acorns per hectare, or, in the case of planting acorns, to plant from 400 to 600 kg of acorns per hectare, and in the case of planting oak saplings, it is necessary to plant 10,000 to 15,000 seedlings per hectare. Forestry and Compensation Price List (2015) shows that the cost of growing a pedunculate oak stand, according to the method of costs per hectare, amounts to HRK 82,555/ha (10,937 €/ha). According to data collected at micro-depression site, the survival of tree saplings is four times better on plots that are clear from the logging residue. Even though there was a less difference in quantity of the saplings between the plot with no residue (N=471) and plot covered with felling debris (N=368) at micro-elevation site, the share of damaged saplings at residue free site (25.7%) and the average share on plots covered with residue (51.4%) show distinctive difference (Fig. 2).
Considering the rodent damage on tree species found at both research sites, damaged stem was the most common problem among all three groups of tree species included in this research (pedunculate oak, narrow-leaved ash, other deciduous species). Considering the damaged saplings of all the inspected species at the site level, 59.3% account for stem damage and 40.7% for root damage at micro-depression site, while at micro-elevation site that ratio was 91.8% and 8.2% in favour of stem damage.
The fact that 44.7% of damaged pedunculate oak saplings at micro-depression site were irretrievably lost, as their root was eaten by rodents, brings about the need for »a new approach« in prevention of rodent damage. It all becomes even more clear having in mind that rodent control methods (i.e. rodenticide use, A.I. zinc phosphide, aver. 3 kg/ha) were applied at this research site at the time of this research. These findings also speak in favour of the previous work by numerous authors (Liang 1982, Liang et al. 1984, Lapasha and Powell 1994, Zhang 1996, Huang and Feng 1998, Qi et al. 1998, Singleton 1999, Koehler et al. 1990), who stated that rodenticide use has very short-term positive effects in reducing the number of small rodents, while dead individuals are replaced quickly by new ones from nearby area. Despite the drier conditions at the micro-elevation site, where even more severe rodent damage could be expected, 9.9% of all damaged saplings lost their roots because of rodents. Other damaged deciduous species suffered permanent damage on 11.9% of the saplings at micro-depression site.
During the last 40 years, an average forest area exposed to rodent damage in Croatian state forests has been extended to almost 3000 hectares per year (PPSF 1980–2020), but it also shows a relatively fast growing trend. For example, rodent damage was established on 2000 ha/year from 1981 to 2000, while from 2000 to 2020 it almost reached 4000 ha. More recently, from 2011 to 2020, the average rodent damage in Croatian state forests was established on almost 5000 ha per year. Another thing that is starting to show changes in the pattern is the dynamics of the rodent outbreaks. Until recently, common rodent species of Croatian inland forests[1] were reaching their density peaks (outbreaks) every 3–4 years on average, which corresponded to similar dynamics recorded in other European countries (Hansson and Henttonen 1985, Delattre et al. 1992, Tkadlec and Stenseth 2001, Lambin et al. 2006, Ims et al. 2008). However, during the last decade, four outbreaks were recorded in Croatian forests (PPSF 1980–2020), which possibly indicates the shortening, or the attenuation, or even gradually losing the cycles and could be a result of climate change (Cornulier 2013). Growing trends of rodent numbers could partially be explained by the findings of Tews et al. (2009), who state that shorter-lived species appear to be better able to adapt under climate changes. All of these trends unfortunately have the most severe impact on and present serious challenge to pedunculate oak and narrow-leaved ash forest ecosystems, as they have become more vulnerable in the last decades and are nowadays known as the most sensitive species of lowland forests in Croatia due to microclimatic and macroclimatic changes and the unfavourable interaction of a whole series of anthropogenic, abiotic and biotic factors (Kalafadžić et al. 1990, Prpić et al. 1994, Prpić 1996, Gradečki 1999, Matić 2009, Prpić 2003, Potočić et al. 2017, Bakys et al. 2009, Kräutler and Kirisits 2012, Gross et al. 2014). Applying the IPM standards in rodent management also implies the need to take into consideration that rodent dynamics and outbreak cycles depend on numerous factors (e.g. age and gender structure, social relations, interspecies competition, genetic predisposition, disease, mortality, climatic and habitat conditions, duration of floods, food supply, masting, forest management, wood residues, predatory species, etc.) (Flowerdew and Gardner 1978, Andorić 1981, Pietsch 1978, Henttonen 2000, Gliwicz 1980, Huitu et al. 2013, Solonen 2004, Solonen 2001, Marsh and Harris 2000, Bujalska 1981, Bryja et al. 2002, Tkadlec and Zejda 1998, Andreassen et al. 2021). It is also important to understand that most of them are beyond our control. Singleton (1999), while stressing some of the negative aspects of IPM (like the over/use of rodenticides), pointed out that moving towards an »Ecologically Based Rodent Management« means the need to better understand rodent biology and social aspects of our common rodent species. In years ahead, we will be probably facing unpredictable climate effects and potentially very variable small rodent dynamics. Keeping that in mind, it is important to continue with rodent monitoring that we started 40 years ago – and to strive for further improvements – while long term monitoring is crucial to determine the stability and sustainability of wildlife populations and their changes (Krebs 2019). It is also necessary to shift the focus from the use of rodenticides to the use of rodent control methods that would meet the ecological standards of FSC certificate in Croatian forestry. One step in that direction may be the application of a residue management that would serve as an applicable prevention method and also provide better conditions for a forest young-growth, free of rodents.
5. References
Andreassen, H.P., Sundell, J., Fraucke, E., Halle, S., Haapakoski, M., Henttonen, H., Huitu, O., Jacob, J., Johnsen, K., Koskela, E., Luque‑Larena, J.J., Lecomte, N., Leirs, H., Mariën, J., Neby, M., Rätti, O., Sievert, T., Singleton, G.R., Cann, J., Broecke, B.V., Ylönen, H., 2021: Population cycles and outbreaks of small rodents: ten essential questions we still need to solve. Oecologia 195(3): 601–622. https://doi.org/10.1007/s00442-020-04810-w
Androić, M., 1981: Priručnik Izvještajne i Dijagnostičko – prognozne službe zaštite šuma, savez inženjera i tehničara šumarstva i industrije za preradu drveta Jugoslavije, 319–335, Beograd.
Anić, I., 2007: Uzgajanje šuma I. Interna skripta, Šumarski fakultet Sveučilišta u Zagrebu, 18–38 p.
Bakys, R., Vasaitis, R., Barklund, P., Ihrmark, K., Stenlid, J., 2009: Investigations concerning the role of Chalara fraxinea in declining Fraxinus excelsior. Plant Pathol 58(2): 284–292. https://doi.org/10.1111/j.1365-3059.2008.01977.x
Bartmann, R.M., White, G.C., Carpenter, L.H., 1992: Compensatory mortality in a Colorado mule deer population. Wildlife Monographs 121: 1–39.
Bäumler, W., 1990: Mäuseschäden in Forstkulturen. Anz. Schädlingsk. Pfl anzensch. Umweltsch 63: 52–55.
Belyakov, N., 2019: Bioenergy. In Sustainable Power Generation – Current Status, Future Challenges, and Perspectives. Academic Press, 593 p.
Birkedal, M., Fischer, A., Karlsson, M., Löf, M., Madsen, P., 2009: Rodent impact on establishment of direct-seeded Fagus sylvatica, Quercus robur and Quercus petraea on forest land. Scandinavian Journal of Forest Research 24(4): 298–307. https://doi.org/10.1080/02827580903055125
Bryja, J., Heroldová, M., Zejda, J., 2002: Effects of deforestation on structure and diversity of small mammal communities in the Moravskoslezske Beskydy Mts (Czech Republic). Acta Theriologica 47(3): 295–306. https://doi.org/10.1007/BF03194148
Bujalska, G., 1981: Formation of seks structure in populations of bank vole (Clethrionomys glareolus Schreber 1780). Wiad. Ekol. 27: 37–48.
Cornulier, T., Yoccoz, N.G., Bretagnolle, V., 2013: Europe-wide dampening of population cycles in keystone herbivores. Science 340(6128): 63–66. https://doi.org/10.1126/science.1228992
Crnković, D., 1982: Kontrola brojnosti i suzbijanje miševa na području SŠGO "Slavonska šuma" Vinkovci. Zbornik radova, 285–287 p.
Davies, R.J., Pepper, H.W., 1993: Protecting trees from field voles. Arboriculture Research Note, AAIS 74: 1–3.
Delany, M.J., 1974: The ecology of small mammals. Studies in biology, Edward Arnold, London, 60 p.
Delattre, P., Giraudoux, P., Baudry, J., Musard, P., Tussaint, M., Truchetet, D., Stahl, P., Poule, M.L., Artois, M., Damange, J.-P., Quéré, J.-P., 1992: Land use patterns and types of common vole (Microtus arvalis) population kinetics. Agric. Ecosyst. Environ. 39(3–4): 153–169. https://doi.org/10.1016/0167-8809(92)90051-C
EPA, 2013: Integrated Pest Management (IPM) Principles. Title on Site: http://www.epa.gov/pesticides/factsheets/ ipm.htm. Previously available.
Erlinge, S., Göransson, G., Hansson, L., Högstedt, G., Liberg, O., Nilsson, I.N., Nilsson, T., von Schantz, T., Sylven, M., 1983: Predation as a regulating factor on small rodent populations in southern Sweden. Oikos 40: 36–52.
Flowerdew, J.R., Gardner, G., 1978: Small Rodent Populations and Food Supply in a Derbyshire Ashwood. Journal of Animal Ecology 47(3): :725–740. https://doi.org/10.2307/3667
Glavaš, S., 2017: Šumski red – praktični primjeri. Lecture. Hrvatska komora inženjera šumarstva i drvne tehnologije, Zagreb.
Gill, R.M.A., 1992: A review of damage by mammals in north temperate forests. 2. Small mammals. Forestry 65(3) :281–308. https://doi.org/10.1093/forestry/65.3.281
Gliwicz, J., 1980: Ecological aspect of synurbanization of the striped field mouse, Apodemus agrarius. Wiadomosci Ekologiczne 26: 117–124.
Gradečki, M., 1999: Uloga i značaj kakvoće sjemena kod njegove uporabe. Rad. Šum. Inst. Jastreb. 34(1): 95–102.
Gross, A., Holdenrieder, O., Pautasso, M., Queloz, V., Sieber, T.N., 2014: Hymenoscyphus pseudoalbidus, the causal agent of European ash dieback. Mol. Plant Pathol. 15(1): 5–21. https://doi.org/10.1111/mpp.12073
Hansson, L., Zejda, J., 1977: Plant damage by bank voles (Clethrionomys glareolus Schreb.) and related species in Europe. EPPO Bull. 7(2): 223–242. https://doi.org/10.1111/j.1365-2338.1977.tb02725.x
Hansson, L., 1982: Experiments on habitat selection in voles: implications for the inverse distribution of two common European species. Oecologia 52(2): 246–252. https://doi.org/10.1007/BF00363844
Hansson, L., Henttonen, H., 1985: Gradients in density variations of small rodents: the importance of latitude and snow cover. Oecologia 67(3): 394–402.
Henttonen, H., 2000: Long-term dynamics of bank vole C. glareolus at Pallasjärvi, northern Finnish taiga. Pol. J. Ecol. 48: 87–96.
Heroldova, M., Suchomel, J., Purchart, L., Homolka, M., Kamler, J., 2007: Small forest rodents: an important factor in the regeneration of forest stands. Beskydy 20: 217–220.
Holland, E.P., James, A., Ruscoe, W.A., 2015: Climate-based models for pulsed resources improve predictability of consumer population dynamics: outbreaks of house mice in forest ecosystems. PLoS One 10(3): e0119139. https://doi.org/10.1371/journ al.pone.01191 39
Huang, X.Q., Feng, Z.Y., 1998: Ecology and management strategies for Rattus losea. U: Zhibin Zhang and Zuwang Wang, (ur.), Ecology and management strategies of rodent pests in agriculture. Beijing, China Ocean Press, 178–194.
Huitu, O., Koivula, M., Korpimäki, E., Klemola, T., Norrdahl, K., 2003: Winter food supply limits growth of northern vole populations in the absence of predation. Ecology 84(8): 2108–2118. https://doi.org/10.1890/02-0040
Huitu, O., Rousi, M., Henttonen, M., 2013: Integration of vole management in boreal silvicultural practices. Pest Management Science, Special Issue: 8th European Vertebrate Pest Management Conference 69(3): 355–361. https://doi.org/10.1002/ps.3264
Hytönen, J., Jylhä, P., 2005: Effects of competing vegetation and post-planting weed control on the mortality, growth and vole damages to Betula pendula planted on former agricultural land. Silva Fenn. 39(3): 365–380. https://doi.org/10.14214/sf.374
Ims, R.A, Henden, J.-A., Killengreen, S.T., 2008: Collapsing population cycles. Tree 23(2): 79–86. https://doi.org/10.1016/j.tree.2007.10.010
Jacob, J., Halle, S., 2001: The importance of land management for population parameters and spatial behaviour in common voles (Microtus arvalis). Advances in vertebrate pest management. II. Fürth: Filander Verlag, 319–330.
Jacob, J., Tkadlec, E., 2010: Rodent outbreaks in Europe: dynamics and damage. In: Singleton, G.R., Belmain, S.R., Brown, P.R., Hardy,B., 2010. Rodent outbreaks: ecology and impacts. Los Baños (Philippines): International Rice Research Institute: 289 p.
Kalafadžić, Z., Kušan, V., 1990: Oštećenje šumskog drveća i sastojina. Šum. list 114(11–12): 517–526.
King, C.M., 1985: Interactions between woodland rodents and their predators. Symp. Zool. Soc. Lond. 55: 219–247.
Koehler, A.E., Marsh, R.E., Salmon, T.P., 1990: Frightnening methods and devices/stimuli to prevent mammal damage – a review. Proceedings of the Fourteenth Vertebrate Pest Conference 1990 Vertebrate Pest Conference Proceedings collection: 167–173.
Kolb, M., Weisshaar, R., 2005: Prognose und Bekämpfung von Wühlmausarten. AFZ-Der Wald 1: 25–27.
Kräutler, K., Kirisits, T., 2012: The ash dieback pathogen Hymenoscyphus pseudoalbidus is asociated with leaf symptoms on ash species (Fraxinus spp.). J. Agric. Ext. Rural Dev. 4(9): 261–265. https://doi.org/10.5897/JAERD12.065
Krebs, C.J., Boonstra, R., Gilbert, B.S., Kenney, A.J., Boutin, S., 2019: Impact of climate change on the small mammal community of the Yukon boreal forest. Integrative Zoology 14(6): 528–541. https://doi.org/10.1111/1749-4877.12397
Križančić, M., 2012: Štete od sitnih glodavaca na području šumarije Draganić u 2007/2008. godini, Diplomski rad, Šumarski fakultet Sveučilišta u Zagrebu, 43–45.
Lambert, D., 1985: The Field Guide to Prehistoric Life, New York: Facts on File Publications, 1985.
Lambin, X., Bretagnolle, V., Yoccoz, N.G., 2006: Vole population cycles in northern and southern Europe: Is there a need for different explanations for single pattern? J. Anim. Ecol. 75(2): 340–349. https://doi.org/10.1111/j.1365-2656.2006.01051.x
Lapasha, D.G., Powell, R.A., 1994: Pine vole (Microtus pinetorum) movement toward areas in apple orchards with reduced populations. Journal of Horticultural Science 69(6): 1077–1082. https://doi.org/10.1080/00221589.1994.11516547
Liang, J.R, Zhou, L., Wang, Z.W., Song, R.Y., 1984: Mathematical model of population recovery of Chinese Zokor (Myospalux tanieri) and plateau pika (Ochotona curzoniae). Acta Ecologica Sinica 4: 1–11.
Liang, J.R., 1982: Population recovery of Chinese zokor (Myospalax fantanieri) and plateau pika (Ociatona curzaniae). In: Wuping Xia, cd., Alpine Gansu, Gansu People's Publishing Press, 93–100.
Margaletić J., 1997: Mišoliki glodavci i njihova štetnost u Turopoljskom Lugu i šumama Hrvatske. Magistarski rad, Šumarski fakultet Sveučilišta u Zagrebu, 1997: 20., 25., 26., 91., 80–82 p.
Margaletić, J., 2003: Promjene u sastavu šumskih populacija sitnih glodavaca nakon mehaničkih zahvata u staništu, Seminar DDD i ZUPP 2003 – stručnost prije svega, Poreč, 12. do 14. ožujaka: djelatnost dezinfekcije, dezinsekcije, deratizacije i zaštite uskladištenih poljoprivrednih proizvod : zbornik radova, Korunić, Zlatko (ur.). Zagreb: Korunić, 117–122 p.
Margaletić, J., Božić, M., Jazbec, A., Čavlović, J., Lukić, N., 2007: Small rodents as the cause of decrease in young narrow-leaved ash tree growth (Fraxinus angustifolia Vahl). Ekologia 26(4): 371–380.
Margaletić, J., Glavaš, M., Bäumler, W., 2002: The development of mice and voles in an oak forest with a surplus of acorns. Anzeiger für Schädlingskunde – Journal of Pest Science 75(4): 95–98.
Marsh, A.C., Harris, S.S., 2000: Partitioning of woodland habitat resources by two sympatric species of Apodemus: lessons for the conservation of the yellow – necked mouse (A. flavicollis) in Britain. Biol. Cons. 92(3): 275–283.
Matić , S., 2009: Gospodarenje šumama hrasta lužnjaka (Quercus robur L.) u promijenjenim stanišnim i strukturnim uvjetima. In: S. Matić, I. Anić, (ur.), Šume hrasta lužnjaka u promijenjenim stanišnim i gospodarskim uvjetima, Hrvatska akademija znanosti i umjetnosti, Zagreb, 1–22 p.
Meerburg, B.G., Singleton, G.R., Kijlstra, A., 2009: Rodent-borne diseases and their risks for public health. Crit Rev Microbiol 35(3): 221–270. https ://doi.org/10.1080/10408 41090 29898 37
Mujezinović, O., 2010: Sitni glodari u šumskim ekosistemima Bosne i Hercegovine. Disertacija, Univerzitet u Sarajevu, Šumarski fakultet, Sarajevo, 110–113 p.
Niemeyer, H., Haase, R., 2003: The importance of voles in afforestation of farmland. Forst und Holz 58 :26-31.
Nilsson, U., Örlander, G., 1999: Vegetation management on grass-dominated clearcuts planted with Norway spruce in southern Sweden. Can J For Res 29(7): 1015–1026. https://doi.org/10.1139/x99-071
Ostfeld, R.S., Jones, C.G., Wolff, J.O., 1996: Of mice and mast. Bioscience 46(5): 323–330. https://doi.org/10.2307/1312946
Pelz, H.J., 2003: Current approaches towards environmentally benign prevention of vole damage in Europe. In: Grant, R., Singleton, Lyn, A., Hinds, Charles, J., Krebs, Dave, M., Spratt, 2003. Rats, mice and people: rodent biology and management. ACIAR Monograph No. 96, 564 p.
Pergola, M.T., Saulino, L., Castellaneta, M., Rita, A., Pecora, G., Cozzi, M., Moretti, N., Pericolo, O., Pierangeli, D., Romano, S., Viccaro, M., Ripullone, F., 2022: Towards sustainable management of forest residues in the southern Apennine Mediterranean mountain forests: a scenario-based approach. Annals of Forest Science 79(1): 1–13. https://doi.org/10.1186/s13595-022-01128-w
Pietsch, M., 1978: Four-year studies on the population dynamics and home range the wood mouse (Apodemus sylvaticus), bank vole (Clethrionomys glareolus) and field vole (Microtus agrestis) in a young conifer plantationin the western Ruhr. Zeitschrift fur Angewandte Zoologie 65: 461–475.
Potočić, N., Seletković, I., Jakovljević, T., Marjanović, H., Indir, K., Medak, J., Lacković, N., Ognjenović, M., Laslo, A., 2017: Oštećenost šumskih ekosustava Republike Hrvatske – izvješće za 2016. godinu. Nacionalni koordinacijski centar za procjenu i motrenje utjecaja atmosferskog onečišćenja i drugih čimbenika na šumske ekosustave, Hrvatski šumarski institut, Jastrebarsko.
PPSF, plant protection survey in forestry: 1980–2016, Croatian Mnistry of Agriculture, Croatian Forest Research Institute (CFRI).
Prpić, B., 1996: Propadanje šuma hrasta lužnjaka, Hrast lužnjak u Hrvatskoj, Hrvatska akademija znanosti i umjetnosti, Zagreb, 273 p.
Prpić, B., 2003: Utjecaj tehničkih zahvata u prostoru na nizinske šume. Šum. list 127(11–12): 230–235.
Prpić, B., Vranković, A., Rauš, Đ., Matić, S., Pranjić, A., Meštrović, Š., 1994: Utjecaj ekoloških i gospodarskih činilaca na sušenje hrasta lužnjaka u gospodarskoj jedinici Kalje šumskog gospodarstva Sisak. Glas. šum. pokuse 30: 361–419.
Qi, G'x'., Yao, W.L., Wang, J., Yang, B., 1998: Research on population dynamics and control strategies for rodents in cities and towns of southern China. Acta Therio-Iogica Sinica 18: 226–230.
Ring, E., Mikael, A., Linnea, H., Gunnar, J. Högbom, L., 2022: Logging Mats and Logging Residue as Ground Protection during Forwarder Traffic along Till Hillslopes. Croat J For Eng 42(3): 445-462. https://doi.org/10.5552/crojfe.2021.875
Rooney, S., Hayden, T.J., 2002: Forest mammals: management and control. In: Singleton, G.R., Belmain, S., Brown, P.R., et al: 2010. Impacts of rodent outbreaks on food security in Asia. Wildl Res 37(5): 355–359. https://doi.org/10.1071/WR10084
Singleton, C.R., Hinds, L.A., Leirs, H., Zhang, Z., 1999: Ecologically-based management of rodent pests. ACIAR Monograph No. 59. 494 p.
Singleton, G.R., Herwig, L., Hinds, L.A., Zhang, Z., 1999: Ecologically-based Management of Rodent Pests–Re-evaluating Our Approach to an Old Problem. In: Singleton, G., Hinds, L., Leirs, H., Zhang, Z., ed. 1999. Ecologically-based management of rodent pests. ACIAR Monograph No. 59.
Solonen, T., 2001: Has owl availability deteriorated due to mild winters in southern Finland? Linnut 36: 6–9.
Solonen, T., 2004: Are vole-eating owls affected by mild winters in southern Finland? Ornis Fennica 81(2): 65–74.
Stapić, L., 2017: Utjecaj korovske vegetacije na pojavu šteta od sitnih glodavaca. Diplomski rad, Šumarski fakultet Sveučilišta u Zagrebu, 13–29 p.
Suchomel, J., 2008: A contribution towards the knowledge of the effect of small mammals on the regeneration of forest trees in selected stands of the Kelec upland (Czech Republic). Acta Univ. Agric. Silvicult. Mendelianea Brunenis 1: 267–270.
Sutton, R.F., 1993: Mounding site preparation: A review of European and North American experience. New Forest 7(2): 151–192. https://doi.org/10.1007/BF00034198
Tews, J., Peter, L.F., Fast, M., 2009: Climate change effects on wildlife populations. In: Wildlife: Destruction, Conservation and Biodiversity, Editors: J.D. Harris and P.L. Brown, Nova Science Publishers, Inc., 239–252 p.
Thompson, D.G., Pitt, D.G., 2003: A review of Canadian forest vegetation management research and practice. Ann For Sci 60(7): 559–572. https://doi.org/10.1051/forest:2003060
Tkadlec, E., Zejda, J., 1998: Small rodent population fuctuations: The effects of age structure and seasonality. Evolutionary Ecology 12(2): 191–210. https://doi.org/10.1023/A:1006583713042
Tkadlec, E., Stenseth, N.C., 2001: A new geographical gradient in vole population dynamics. Proc. R. Soc. Lond. B, 268(1476): 1547–1552. https://doi.org/10.1098/rspb.2001.1694
Ugrenović, A., Benić, R., 1957: Eksploatacija šuma. Poljoprivredni nakladni zavod, 288 p.
Vajda, Z., 1974: Nauka o zaštiti šuma. Izdanje Školska knjiga, Zagreb, 20 p.
Vucelja, M., Margaletić, J., Bjedov, L., Moro, M., Šango, M., 2014: Štete od sitnih glodavaca na stabljici i korijenu hrasta lužnjaka (Quercus robur, L.). Šum. list, 138(5–6): 283–291.
Vucelja, M., 2013: Zaštita od glodavaca (Rodentia, Murinae, Arvicolinae) u šumama hrasta lužnjaka (Quercus robur L.) – integrirani pristup i zoonotički aspekt. Doktorska disertacija, Šumarski fakultet Sveučilišta u Zagrebu, 25–210.
Väätäinen, K., Anttila, P., Eliasson, L., Johanna, E., Juha, L., Robert, P., Johanna, R., 2021: Roundwood and Biomass Logistics in Finland and Sweden. Croat J For Eng 42(1): 39–61. https://doi.org/10.5552/crojfe.2021.803
Vucelja, M., Bjedov, L., Margaletić, J., 2018: Unapređenje metodologije sustavnog monitoringa sitnih glodavaca i zaštite od njihova štetnog utjecaja u poplavnim šumama Hrvatske. In »Ekologija i obnova poplavnih šuma Posavine«, urednik Milan Oršanić, Zagreb 2019.
Walker, L.A., Turk, A., Long, S., Weinburg, C.L., Best, J., Shore, R.F., 2008: Second generation anticoagulant rodenticides in tawny owls (Strix aluco) from Great Britain. Science of The Total Environment 392(1): 93–98. https://doi.org/10.1016/j.scitotenv.2007.10.061
Ward, D., 1993: Protective measures against the bank vole. Irish Timber Forestry 2: 22 p.
Zečić, Ž., Vusić, D., 2016: Šumski proizvodi (skripta). Šumarski fakultet Sveučilišta u Zagrebu, Zagreb, 171–173 p.
Zhang, Z.B., 1996: Techniques and strategies for rodent control by contraception. U: Zuwang, W., Zhibin, Z. (ur.), Theory and practice of rodent pest management. Beijing, Science Press, 367–368.
© 2022 by the authors. Submitted for possible open access publication under the
terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Authors’ addresses:
Assist. prof. Marko Vucelja, PhD
e-mail: marko.vucelja@sumfak.unizg.hr
Linda Bjedov, PhD *
e-mail: linda.bjedov@sumfak.unizg.hr
Assist. prof. Kristijan Tomljanović, PhD
e-mail: kristijan.tomljanovic@sumfak.unizg.hr
Jelena Kranjec Orlović, PhD
e-mail: jelena.kranjec@sumfak.unizg.hr
Prof. Josip Margaletić, PhD
e-mail: josip.margaletic@sumfak.unizg.hr
University of Zagreb
Faculty of Forestry and Wood Technology
Department of Forest Protection and Wildlife Management
Svetošimunska 23
10 000 Zagreb
CROATIA
Marko Boljfetić, Msc
e-mail: mboljfetic@oikon.hr
Oikon Ltd. – Institute of Applied Ecology
Trg senjskih uskoka 1–2
10 020 Zagreb
CROATIA
Mislav Matijević, Msc
e-mail: mislav.matijevic@hrsume.hr
Hrvatske šume – limited liability company
Ulica kneza Branimira 1
10 000 Zagreb
CROATIA
* Corresponding author
Received: February 18, 2022
Accepted: September 28, 2022
Original scientific paper
[1] Apodemus spp. Kaup 1829, Clethrionomys (sin. Myodes) glarelous spp. Tilesius 1850 and Microtus spp. Schrank 1798)

